Specific roles for the PI3K and the MEK-ERK pathway in IGF-1-stimulated chemotaxis, VEGF secretion and proliferation of multiple myeloma cells: study in the 5T33MM model - PubMed (original) (raw)
Specific roles for the PI3K and the MEK-ERK pathway in IGF-1-stimulated chemotaxis, VEGF secretion and proliferation of multiple myeloma cells: study in the 5T33MM model
E Menu et al. Br J Cancer. 2004.
Abstract
Insulin-like growth factor-1 (IGF-1) has been described as an important factor in proliferation, cell survival and migration of multiple myeloma (MM) cells. Angiogenesis correlates with development and prognosis of the MM disease. Vascular endothelial growth factor (VEGF) is one of the prominent factors involved in this process. The different functions of IGF-1 were investigated in the 5TMM mouse model with emphasis on proliferation, migration and VEGF secretion, and the signalling pathways involved. Western Blot analysis revealed that ERK1/2 and Akt (PKB) were activated after IGF-1 stimulation. The activation of ERK1/2 was reduced by the PI3K inhibitor Wortmannin, implying that the PI3K pathway is involved in its activation. Insulin-like growth factor-1 induced an increase in DNA synthesis in MM cells, which was mediated by a PI3K/Akt-MEK/ERK pathway. Insulin-like growth factor-1 enhanced F-actin assembly and this process was only PI3K mediated. Stimulation by IGF-1 of VEGF production was reduced by PD98059, indicating that only the MEK-ERK pathway is involved in IGF-1-stimulated VEGF production. In conclusion, IGF-1 mediates its multiple effects on MM cells through different signal transduction pathways. In the future, we can study the potential in vivo effects of IGF-1 inhibition on tumour growth and angiogenesis in MM.
Figures
Figure 1
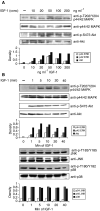
Activation of the MEK–ERK and the PI3K pathway after IGF-1 stimulation. (A) 5T33MM cells were stimulated with increasing concentrations of IGF-1 for 10 min. (B) 5T33MM cells were stimulated during different periods of time with 100 ng ml−1 IGF-1. Equivalent amounts of lysates were immunoblotted with anti-P-ERK1/2 (first panel), P-Akt (third panel), P-Jnk (fifth panel) and P-p38 (seventh panel) and reblotted with anti-ERK1/2 (second panel), anti-Akt (fourth panel), anti-JNK (sixth panel) and anti-p38 (eigth panel) to confirm equal loading. One experiment representative of four is shown.
Figure 2
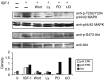
Crosstalk between the PI3K pathway and the MEK–ERK pathway. The MEK inhibitors PD98059 and UO126 abolish stimulation by IGF-1 of ERK phosphoryation but have no influence on the phosphorylation of Akt (first and third panel). The inhibitors of the PI3K pathway Wortmannin and Ly294002, on the other hand, inhibit the phosphorylation of Akt (PKB), confirming that Akt becomes phosphorylated through activation of the PI3K pathway (third panel), but also reduces the phosphorylation of ERK1 and 2 (first panel). The cells were stimulated with 100 ng ml−1 IGF-1 for 10 min and lysates were treated as in the previous figure. One experiment representative of four is shown.
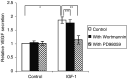
Figure 3
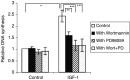
IGF-1 induced DNA synthesis. For the thymidine incorporation assays, the MM cells were incubated with RPMI in the absence or the presence of 10 ng ml−1 IGF-1. Before stimulation with or without IGF-1, the cells were preincubated for 30 min with Wortmannin (100 n
M
), PD98059 (20 μ
M
) or both where indicated. Mean values±s.d. for four independent experiments are shown. (*: P<0.01 vs control, **: P<0.01 vs IGF-1, ***: P<0.01 vs IGF-1).
Figure 4
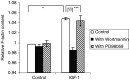
IGF-1 induced F-actin assembly. The F-actin content of the MM cells was measured by FACS analysis. The mean fluorescence intensity is shown as the relative value compared to unstimulated cells. The cells were stimulated with or without 100ng ml−1 IGF-1, after a 30 min incubation with Wortmannin (100 n
M
) or PD98059 (20 μ
M
) where indicated. The cells were then labelled with phalloidin FITC. Mean values±s.d. for three independent experiments are shown (*: P<0.02 _vs_ control, **: _P_<0.04, ***: _P_>0.05 vs IGF-1).
Figure 5
Stimulation by IGF-1 of VEGF secretion. The 5T33MM cells were stimulated with or without 100 ng ml−1 IGF-1 for 24 h, after a 30 min incubation with Wortmannin (100 n
M
) or PD98059 (20 μ
M
) where indicated. Concentrations of VEGF are shown relative to unstimulated cells. The maximum IGF-1-stimulated VEGF secretion reaches 250pg ml−1. Mean values±s.d. for three independent experiments are shown (*: P<0.01 _vs_ control, **: _P_<0.01, ***: _P_>0.05 vs 100 ng ml−1 IGF-1).
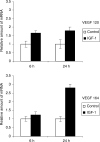
Figure 6
IGF-1 stimulates VEGF mRNA expression. The MM cells were stimulated with 100 ng ml−1 IGF-1 for 6 or 24 h. The relative amount of VEGF in the IGF-1-stimulated samples compared to the control samples is shown. Error bars represent s.d. in the experiment. One experiment representing three is illustrated, P<0.05.
Similar articles
- Downmodulation of ERK protein kinase activity inhibits VEGF secretion by human myeloma cells and myeloma-induced angiogenesis.
Giuliani N, Lunghi P, Morandi F, Colla S, Bonomini S, Hojden M, Rizzoli V, Bonati A. Giuliani N, et al. Leukemia. 2004 Mar;18(3):628-35. doi: 10.1038/sj.leu.2403269. Leukemia. 2004. PMID: 14737074 - Insulin-like growth factor I-mediated protection from rapamycin-induced apoptosis is independent of Ras-Erk1-Erk2 and phosphatidylinositol 3'-kinase-Akt signaling pathways.
Thimmaiah KN, Easton J, Huang S, Veverka KA, Germain GS, Harwood FC, Houghton PJ. Thimmaiah KN, et al. Cancer Res. 2003 Jan 15;63(2):364-74. Cancer Res. 2003. PMID: 12543789 - Participation of PI3K and ERK1/2 pathways are required for human brain vascular smooth muscle cell migration.
Yang GY, Yao JS, Huey M, Hashimoto T, Young WL. Yang GY, et al. Neurochem Int. 2004 May;44(6):441-6. doi: 10.1016/j.neuint.2003.07.002. Neurochem Int. 2004. PMID: 14687609 - MicroRNAs in Tumor Endothelial Cells: Regulation, Function and Therapeutic Applications.
Gu Y, Becker MA, Müller L, Reuss K, Umlauf F, Tang T, Menger MD, Laschke MW. Gu Y, et al. Cells. 2023 Jun 22;12(13):1692. doi: 10.3390/cells12131692. Cells. 2023. PMID: 37443725 Free PMC article. Review.
Cited by
- Targeting MEK induces myeloma-cell cytotoxicity and inhibits osteoclastogenesis.
Tai YT, Fulciniti M, Hideshima T, Song W, Leiba M, Li XF, Rumizen M, Burger P, Morrison A, Podar K, Chauhan D, Tassone P, Richardson P, Munshi NC, Ghobrial IM, Anderson KC. Tai YT, et al. Blood. 2007 Sep 1;110(5):1656-63. doi: 10.1182/blood-2007-03-081240. Epub 2007 May 17. Blood. 2007. PMID: 17510321 Free PMC article. Retracted. Clinical Trial. - The insulin-like growth factor system in multiple myeloma: diagnostic and therapeutic potential.
Bieghs L, Johnsen HE, Maes K, Menu E, Van Valckenborgh E, Overgaard MT, Nyegaard M, Conover CA, Vanderkerken K, De Bruyne E. Bieghs L, et al. Oncotarget. 2016 Jul 26;7(30):48732-48752. doi: 10.18632/oncotarget.8982. Oncotarget. 2016. PMID: 27129151 Free PMC article. Review. - Type I insulin-like growth factor receptor signaling in hematological malignancies.
Vishwamitra D, George SK, Shi P, Kaseb AO, Amin HM. Vishwamitra D, et al. Oncotarget. 2017 Jan 3;8(1):1814-1844. doi: 10.18632/oncotarget.12123. Oncotarget. 2017. PMID: 27661006 Free PMC article. Review. - Effect of the HDAC inhibitor vorinostat on the osteogenic differentiation of mesenchymal stem cells in vitro and bone formation in vivo.
Xu S, De Veirman K, Evans H, Santini GC, Vande Broek I, Leleu X, De Becker A, Van Camp B, Croucher P, Vanderkerken K, Van Riet I. Xu S, et al. Acta Pharmacol Sin. 2013 May;34(5):699-709. doi: 10.1038/aps.2012.182. Epub 2013 Apr 8. Acta Pharmacol Sin. 2013. PMID: 23564084 Free PMC article. - Cell non-autonomous regulation of cerebrovascular aging processes by the somatotropic axis.
Bickel MA, Csik B, Gulej R, Ungvari A, Nyul-Toth A, Conley SM. Bickel MA, et al. Front Endocrinol (Lausanne). 2023 Jan 23;14:1087053. doi: 10.3389/fendo.2023.1087053. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36755922 Free PMC article. Review.
References
- Asosingh K, Gunthert U, Bakkus MH, De Raeve H, Goes E, Van Riet I, Van Camp B, Vanderkerken K (2000a) In vivo induction of insulin-like growth factor-I receptor and CD44v6 confers homing and adhesion to murine multiple myeloma cells. Cancer Res 60: 3096–3104 - PubMed
- Asosingh K, Radl J, Van Riet I, Van Camp B, Vanderkerken K (2000b) The 5TMM series, a useful in vivo mouse model of human multiple myeloma. Hematol J 1: 351–356 - PubMed
- Bakkus MH, Heirman C, Van Riet I, Van Camp B, Thielemans K (1992) Evidence that multiple myeloma Ig heavy chain VDJ genes contain somatic mutations but show no intraclonal variation. Blood 80: 2326–2335 - PubMed
- Bonacchi A, Romagnani P, Romanelli RG, Efsen E, Annunziato F, Lasagni L, Francalanci M, Serio M, Laffi G, Pinzani M, Gentilini P, Marra F (2001) Signal transduction by the chemokine receptor CXCR3: activation of Ras/ERK, Src, and phosphatidylinositol 3-kinase/Akt controls cell migration and proliferation in human vascular pericytes. J Biol Chem 276: 9945–9954 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous