Interleukin 2 plays a central role in Th2 differentiation - PubMed (original) (raw)
Interleukin 2 plays a central role in Th2 differentiation
Javier Cote-Sierra et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Differentiation of naïve CD4 T cells into T helper (Th) 2 cells requires signaling through the T cell receptor and an appropriate cytokine environment. IL-4 is critical for such Th2 differentiation. We show that IL-2 plays a central role in this process. The effect of IL-2 on Th2 generation does not depend on its cell growth or survival effects. Stat5a(-/-) cells show diminished differentiation to IL-4 production, and forced expression of a constitutively active form of Stat5a replaces the need for IL-2. In vivo IL-2 neutralization inhibits IL-4 production in two models. Studies of restriction enzyme accessibility and binding of Stat5 to chromatin indicate that IL-2 mediates its effect by stabilizing the accessibility of the Il4 gene. Thus, IL-2 plays a critical role in the polarization of naive CD4 T cells to the Th2 phenotype.
Figures
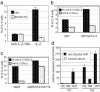
Fig. 1.
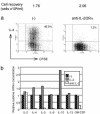
Effect of blocking IL-2 on cytokine production. (a) 5C.C7 CD4 T cells were cultured for 86 h with APC and 0.5 μM PCC peptide under Th2 conditions with anti-CD28. At the end of the priming culture, viable cell numbers, cell division history, and intracellular IL-4 expression were analyzed. (b) DO11.10 cells were cultured under Th2 conditions with immobilized anti-CD3 plus anti-CD28. mRNA levels for various cytokines were measured at 84 h of culture by real-time PCR analysis. GM-CSF, granulocyte/macrophage colony-stimulating factor.
Fig. 2.
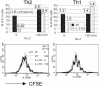
Priming for IL-4 production in IL-2-/- CD4 T cells. IL-2-/- and IL-2+/+ 5C.C7 CD4 T cells were cultured for 86 h with APC and 0.5 μM PCC peptide under Th2 or Th1 conditions plus anti-CD28 with or without 100 units/ml human IL-2 (hIL-2). At the end of the priming culture, viable cell numbers, cell division history, and intracellular IL-4 expression were analyzed.
Fig. 3.
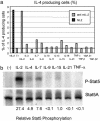
Effect of different cytokines on IL-4 production and Stat5 phosphorylation. (a) 5C.C7 CD4 T cells were cultured with APC and 0.2 μM PCC peptide under Th2 conditions for 94 h, with human (hIL-2) (20 units/ml) or with anti-mouse IL-2 (anti-mIL-2) without or with other cytokines. Intracellular IL-4 expression was analyzed after restimulation. (b) DO11.10 CD4 T cells were cultured under Th2 conditions with immobilized anti-CD3 plus anti-CD28 for 48 h. Cells were washed, cultured overnight in 0.5% FCS containing RPMI medium 1640, washed again, and cultured in serum-free medium for 2 h before cytokine stimulation for 15 min. Phospho-Stat5 and Stat5 were analyzed by immunoblotting. TNF, tumor necrosis factor; TGF, transforming growth factor.
Fig. 4.
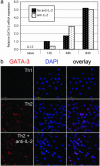
Effect of IL-2 on GATA-3 induction. (a) DO11.10 CD4 T cells were cultured under Th2 conditions with immobilized anti-CD3 plus anti-CD28. GATA-3 mRNA levels were measured by real-time PCR at 0, 12, 48, and 84 h in the presence or absence of anti-IL-2. (b) DO11.10 CD4 T cells were cultured under Th1 and Th2 conditions. Cells were fixed and permeabilized at 72 h, stained with a monoclonal anti-GATA-3, and analyzed by confocal microscopy. DAPI, 4′,6-diamidino-2-phenylindole.
Fig. 5.
Il4 gene accessibility. (a) 5C.C7 CD4 T cells stimulated under Th2 conditions with APC and 0.2 μM PCC peptide. Either IL-2 or anti-IL-2, anti-IL-2Rα, and anti-IL-2Rβ (anti-IL-2) were added. REA at HSII was analyzed by PCR at 86 h. (b) DO11.10 CD4 T cells stimulated with immobilized anti-CD3 plus anti-CD28 under Th2 conditions with either IL-2 or anti IL-2 for 48 h. Cells were washed and cultured under Th2-priming conditions for another 48 h, in the presence of either IL-2 or anti-IL-2, anti- IL-2Rα, and anti-IL-2Rβ. REA was assessed in HSII and HSIII at the outset of culture and at 48 and 96 h of Th2 priming. IL-4-producing capacity was assessed after restimulation. (c) DO11.10 CD4 T cells primed for 48 h under Th2 conditions without exogenous IL-2. Cells were cultured under Th2 conditions for the next 48 h in the presence of IL-2 (IL-4/2), IL-2 plus cyclosporin A (50 ng/ml) (IL-4/2 CsA) or anti-IL-2, anti-IL-2Rα, and anti-IL-2Rβ (IL-4/a2). REA at HS II and IL-4-producing capacity were analyzed at 92 h. (d) DO11.10 cells were cultured under Th2 conditions with immobilized anti-CD3 plus anti-CD28 for 86 h. IL-4-producing cells, identified by cytokine capture, were purified by cell sorting. IL-4+ cells were stimulated for 3 days under Th2 conditions in the presence of IL-2 (IL-4/IL-2) or anti-IL-2, anti-IL-2Rα, and anti-IL-2Rβ (IL-4/a2). REA at HSII and HSIII and IL-4-producing capacity were analyzed at 72 h.
Fig. 6.
Stat5a and IL-4 production. (a) C57BL/6 Stat5a-/- and WT CD4 T cells primed for 86 h with soluble anti-CD3 (0.5 μg/ml) and anti-CD28 (3 μg/ml) under Th-neutral conditions with either anti-IL-2 and anti-IL-2Rα or with added IL-2. KO, knockout. (b and c) 5C.C7 CD4 T cells were activated for 40 h before retrovirus infection. Cells were infected with a GATA3- (b) or a STAT5A1*6- (c) encoding retrovirus and cultured for an additional 3 days under Th2 conditions with IL-2 or anti-IL-2, anti-IL-2Rα, and anti-IL-2Rβ. Intracellular IL-4 expression was analyzed after stimulation in CD4 T cells (a) or STAT5A1*6-, GATA-3-, or control retrovirus-infected cells (b and c). NGFR, nerve growth factor receptor. (d) 5C.C7 CD4 T cells were stimulated under Th1 or Th2 conditions with APC and 1 μM PCC peptide for three rounds. Each round consisted of 4 days priming and 3 days resting in IL-2. Chromatin immunoprecipitation (ChIP) assays were carried out by using anti-Stat5a and control sera. Amounts of immunoprecipitated DNA sequences from HSII, HSIII, and SOCS-3 promoter Stat-binding motif (S3PS) were assessed by real-time PCR. Relative enrichment of the sequences were normalized by using a 3′-UTR t-bet sequence, which does not have a Stat-binding site.
Fig. 7.
In vivo IL-2 neutralization. (a) BALB/c mice implanted with miniosmotic pumps containing 1 μg of ovalbumin in PBS received 5 × 106 purified DO11.10 CD4 T cells. Mice were injected i.p. with anti-IL-2 (S4B6), isotype control, or PBS. Draining lymph node cells were restimulated with ovalbumin peptide and soluble anti-CD28. IL-4 expression, cell division, and number of KJ1-26+ cells were analyzed. Mean IL-4+ KJ1-26 cells in control Ig and anti-IL-2 groups were significantly different (P < 0.0002).
Similar articles
- Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment.
Yamane H, Zhu J, Paul WE. Yamane H, et al. J Exp Med. 2005 Sep 19;202(6):793-804. doi: 10.1084/jem.20051304. J Exp Med. 2005. PMID: 16172258 Free PMC article. - The Runx1 transcription factor inhibits the differentiation of naive CD4+ T cells into the Th2 lineage by repressing GATA3 expression.
Komine O, Hayashi K, Natsume W, Watanabe T, Seki Y, Seki N, Yagi R, Sukzuki W, Tamauchi H, Hozumi K, Habu S, Kubo M, Satake M. Komine O, et al. J Exp Med. 2003 Jul 7;198(1):51-61. doi: 10.1084/jem.20021200. Epub 2003 Jun 30. J Exp Med. 2003. PMID: 12835475 Free PMC article. - An IL-4-independent and CD25-mediated function of c-maf in promoting the production of Th2 cytokines.
Hwang ES, White IA, Ho IC. Hwang ES, et al. Proc Natl Acad Sci U S A. 2002 Oct 1;99(20):13026-30. doi: 10.1073/pnas.202474499. Epub 2002 Sep 23. Proc Natl Acad Sci U S A. 2002. PMID: 12271139 Free PMC article. - An epigenetic view of helper T cell differentiation.
Ansel KM, Lee DU, Rao A. Ansel KM, et al. Nat Immunol. 2003 Jul;4(7):616-23. doi: 10.1038/ni0703-616. Nat Immunol. 2003. PMID: 12830136 Review.
Cited by
- Multi-Source Pathways of T Follicular Helper Cell Differentiation.
Ma X, Nakayamada S. Ma X, et al. Front Immunol. 2021 Feb 25;12:621105. doi: 10.3389/fimmu.2021.621105. eCollection 2021. Front Immunol. 2021. PMID: 33717120 Free PMC article. Review. - CD4⁺T cells: differentiation and functions.
Luckheeram RV, Zhou R, Verma AD, Xia B. Luckheeram RV, et al. Clin Dev Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. Epub 2012 Mar 14. Clin Dev Immunol. 2012. PMID: 22474485 Free PMC article. Review. - T helper 2 and T follicular helper cells: Regulation and function of interleukin-4.
Sahoo A, Wali S, Nurieva R. Sahoo A, et al. Cytokine Growth Factor Rev. 2016 Aug;30:29-37. doi: 10.1016/j.cytogfr.2016.03.011. Epub 2016 Mar 31. Cytokine Growth Factor Rev. 2016. PMID: 27072069 Free PMC article. Review. - The differential expression of IL-4 and IL-13 and its impact on type-2 immunity.
Bao K, Reinhardt RL. Bao K, et al. Cytokine. 2015 Sep;75(1):25-37. doi: 10.1016/j.cyto.2015.05.008. Epub 2015 Jun 11. Cytokine. 2015. PMID: 26073683 Free PMC article. Review. - The role of interleukin-2 during homeostasis and activation of the immune system.
Boyman O, Sprent J. Boyman O, et al. Nat Rev Immunol. 2012 Feb 17;12(3):180-90. doi: 10.1038/nri3156. Nat Rev Immunol. 2012. PMID: 22343569 Review.
References
- Zheng, W. & Flavell, R. A. (1997) Cell 89, 587-596. - PubMed
- Ouyang, W., Ranganath, S. H., Weindel, K., Bhattacharya, D., Murphy, T. L., Sha, W. C. & Murphy, K. M. (1998) Immunity 9, 745-755. - PubMed
- Henkel, G., Weiss, D. L., McCoy, R., Deloughery, T., Tara, D. & Brown, M. A. (1992) J. Immunol. 149, 3239-3246. - PubMed
- Bird, J. J., Brown, D. R., Mullen, A. C., Moskowitz, N. H., Mahowald, M. A., Sider, J. R., Gajewski, T. F., Wang, C. R. & Reiner, S. L. (1998) Immunity 9, 229-237. - PubMed
- Takemoto, N., Koyano-Nakagawa, N., Yokota, T., Arai, N., Miyatake, S. & Arai, K. (1998) Int. Immunol. 10, 1981-1985. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous