A nuclear translation-like factor eIF4AIII is recruited to the mRNA during splicing and functions in nonsense-mediated decay - PubMed (original) (raw)
A nuclear translation-like factor eIF4AIII is recruited to the mRNA during splicing and functions in nonsense-mediated decay
Maria A Ferraiuolo et al. Proc Natl Acad Sci U S A. 2004.
Abstract
In eukaryotes, a surveillance mechanism known as nonsense-mediated decay (NMD) degrades the mRNA when a premature-termination codon (PTC) is present. NMD requires translation to read the frame of the mRNA and detect the PTC. During pre-mRNA splicing, the exon-exon junction complex (EJC) is recruited to a region 20-24 nt upstream of the exon junction on the mature mRNA. The presence of a PTC upstream from the EJC elicits NMD. Eukaryotic initiation factor 4A (eIF4A) III is a nuclear protein that interacts physically or functionally with translation initiation factors eIF4G and eIF4B, respectively, and shares strikingly high identity with the initiation factors eIF4AI/II. Here we show that siRNA against eIF4AIII, but not against eIF4AI/II, inhibits NMD. Moreover, eIF4AIII, but not eIF4AI, is specifically recruited to the EJC during splicing. The observations that eIF4AIII is loaded onto the mRNA during splicing in the nucleus, has properties related to a translation initiation factor, and functions in NMD raises the possibility that eIF4AIII substitutes for eIF4AI/II during NMD.
Figures
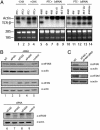
Fig. 1.
RNAi against eIF4AIII impairs NMD. (A) Northern blot analysis of poly(A) RNA hybridized with Vβ8.1 probe (23). Lanes 1 and 2 represent stable cell lines expressing wild-type TCR-β minigene and PTC-containing TCR-β minigene, respectively. Cycloheximide treatment in both cell lines was performed for 2 h at 37°C (100 μg/ml final) (lanes 3 and 4). siRNAs were transfected into stable cell lines expressing a PTC-containing TCR-β minigene (PTC+) (lanes 5–9) and wild-type TCR-β minigene (PTC–) (lanes 10–14). β-Actin served as a loading marker. The 28S and 18S ribosomal RNA serve to demonstrate equal loading for eIF4AIII siRNA treated samples because siRNA against eIF4AIII is accompanied by decreased levels of actin due to cell death (see Results). CHX, cycloheximide. (B and C) Western analysis of the levels of translation initiation factors after knockdown. Thirty micrograms of protein extract was resolved by SDS/PAGE. Proteins were quantitated against β-actin (Sigma) as a loading control and the level of protein in negative control (4E-T inv) transfected cells was normalized to 100. NT, nontransfected.
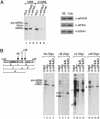
Fig. 2.
eIF4AIII, but not eIF4AI, associates with the EJC on spliced mRNA. (A) eIF4AIII, but not eIF4AI, is associated with spliced mRNA but not with intronless mRNA (Left). Western analysis showing the presence of eIF4AIII, eIF4AI, and tubulin in the nuclear (NE) and cytoplasmic (Cyto) extracts (Right). (B) eIF4AIII associates with the region of the mRNA that contains the EJC. (Left) Schematic of AdML mRNA showing the locations of oligonucleotides. The numbers indicate the middle of each 12-mer oligonucleotide. (The RNA fragments containing the EJC are designated a, c, and e.) (Right) The total reaction after splicing and oligonucleotide-directed RNase H cleavage was used for the immunoprecipitations and is shown in lanes designated input. Immunoprecipitations were carried out with the indicated antibodies after cleavage with each oligonucleotide. The RNA fragments containing the EJC that were immunoprecipitated are indicated (a, c, and e). The RNA fragments that do not contain the EJC are also indicated (b and d). A darker exposure of lanes 9–12 is included to detect fragment d (55 nt) generated with the +0 oligonucleotide (lane 9). The 13-nt RNA fragment generated with the +36 oligonucleotide is not detected.
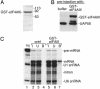
Fig. 3.
eIF4AIII is associated with spliced mRNA in vivo. (A) Coomassie-stained gel showing GST-eIF4AIII used for microinjection into oocyte cytoplasm. (B) Western analysis of Xenopus oocyte nuclei after preinjection showing the presence of UAP56, a known nuclear protein, and recombinant GST-eIF4AIII. (C) Inj indicates the total RNA before injection, and I indicates the RNA species present in the nuclear lysates before the addition of glutathione beads. Total RNA unbound (U) and bound (B) to the beads is shown.
Fig. 4.
eIF4AIII is a shuttling protein. eIF4AIII and 4E-T localization was detected by staining with anti-hemagglutinin antibody (1/1,000 dilution, Covance/Babco) and Texas red-conjugated anti-mouse IgG (Molecular Probes). Human and mouse nuclei are differentiated by staining with Hoechst dye 33258 (Sigma). The mouse nucleus is indicated by an arrow. Leptomycin B (LMB, Sigma) treatments were performed at 5 ng/ml final concentration for 5 h. Images were processed with a Nikon Eclipse E800 microscope at ×60 magnification.
Similar articles
- Mutational analysis of human eIF4AIII identifies regions necessary for exon junction complex formation and nonsense-mediated mRNA decay.
Shibuya T, Tange TØ, Stroupe ME, Moore MJ. Shibuya T, et al. RNA. 2006 Mar;12(3):360-74. doi: 10.1261/rna.2190706. RNA. 2006. PMID: 16495234 Free PMC article. - Human spliceosomal protein CWC22 plays a role in coupling splicing to exon junction complex deposition and nonsense-mediated decay.
Alexandrov A, Colognori D, Shu MD, Steitz JA. Alexandrov A, et al. Proc Natl Acad Sci U S A. 2012 Dec 26;109(52):21313-8. doi: 10.1073/pnas.1219725110. Epub 2012 Dec 10. Proc Natl Acad Sci U S A. 2012. PMID: 23236153 Free PMC article. - eIF4AIII binds spliced mRNA in the exon junction complex and is essential for nonsense-mediated decay.
Shibuya T, Tange TØ, Sonenberg N, Moore MJ. Shibuya T, et al. Nat Struct Mol Biol. 2004 Apr;11(4):346-51. doi: 10.1038/nsmb750. Epub 2004 Mar 21. Nat Struct Mol Biol. 2004. PMID: 15034551 - The long and short of EJC-independent nonsense-mediated RNA decay.
Muñoz O, Lore M, Jagannathan S. Muñoz O, et al. Biochem Soc Trans. 2023 Jun 28;51(3):1121-1129. doi: 10.1042/BST20221131. Biochem Soc Trans. 2023. PMID: 37145092 Review. - New insights into the formation of active nonsense-mediated decay complexes.
Singh G, Lykke-Andersen J. Singh G, et al. Trends Biochem Sci. 2003 Sep;28(9):464-6. doi: 10.1016/S0968-0004(03)00176-2. Trends Biochem Sci. 2003. PMID: 13678954 Review.
Cited by
- The DEAD-box helicase eIF4A: paradigm or the odd one out?
Andreou AZ, Klostermeier D. Andreou AZ, et al. RNA Biol. 2013 Jan;10(1):19-32. doi: 10.4161/rna.21966. Epub 2012 Sep 20. RNA Biol. 2013. PMID: 22995829 Free PMC article. Review. - A role for the eIF4E-binding protein 4E-T in P-body formation and mRNA decay.
Ferraiuolo MA, Basak S, Dostie J, Murray EL, Schoenberg DR, Sonenberg N. Ferraiuolo MA, et al. J Cell Biol. 2005 Sep 12;170(6):913-24. doi: 10.1083/jcb.200504039. J Cell Biol. 2005. PMID: 16157702 Free PMC article. - Assembly, disassembly and recycling: the dynamics of exon junction complexes.
Bono F, Gehring NH. Bono F, et al. RNA Biol. 2011 Jan-Feb;8(1):24-9. doi: 10.4161/rna.8.1.13618. Epub 2011 Jan 1. RNA Biol. 2011. PMID: 21289489 Free PMC article. Review. - Splicing remodels messenger ribonucleoprotein architecture via eIF4A3-dependent and -independent recruitment of exon junction complex components.
Zhang Z, Krainer AR. Zhang Z, et al. Proc Natl Acad Sci U S A. 2007 Jul 10;104(28):11574-9. doi: 10.1073/pnas.0704946104. Epub 2007 Jul 2. Proc Natl Acad Sci U S A. 2007. PMID: 17606899 Free PMC article. - The Physiological Roles of the Exon Junction Complex in Development and Diseases.
Asthana S, Martin H, Rupkey J, Patel S, Yoon J, Keegan A, Mao Y. Asthana S, et al. Cells. 2022 Apr 1;11(7):1192. doi: 10.3390/cells11071192. Cells. 2022. PMID: 35406756 Free PMC article. Review.
References
- Gingras, A. C., Raught, B. & Sonenberg, N. (1999) Annu. Rev. Biochem. 68, 913–963. - PubMed
- Hershey, J. W. B. & Merrick, W. C. (2000) in Translational Control of Gene Expression, ed. Merrick, W. C. (Cold Spring Harbor Lab. Press, Plainview, NY), pp. 33–88.
- Conroy, S. C., Dever, T. E., Owens, C. L. & Merrick, W. C. (1990) Arch. Biochem. Biophys. 282, 363–371. - PubMed
- Weinstein, D. C., Honore, E. & Hemmati-Brivanlou, A. (1997) Development (Cambridge, U.K.) 124, 4235–4242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous