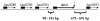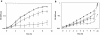The htrA (degP) gene of Listeria monocytogenes 10403S is essential for optimal growth under stress conditions - PubMed (original) (raw)
The htrA (degP) gene of Listeria monocytogenes 10403S is essential for optimal growth under stress conditions
Laura D Wonderling et al. Appl Environ Microbiol. 2004 Apr.
Abstract
This report describes a mutant of Listeria monocytogenes strain 10403S (serotype 1/2a) with a defective response to conditions of high osmolarity, an environment that L. monocytogenes encounters in some ready-to-eat foods. A library of L. monocytogenes clones mutagenized with Tn917 was generated and scored for sensitivity to 4% NaCl in order to identify genes responsible for growth or survival in elevated-NaCl environments. One of the L. monocytogenes Tn917 mutants, designated strain OSM1, was selected, and the gene interrupted by the transposon was sequenced. A BLAST search with the putative translated amino acid sequence indicated that the interrupted gene product was a homolog of htrA (degP), a gene coding for a serine protease identified as a stress response protein in several gram-positive and gram-negative bacteria. An htrA deletion strain, strain LDW1, was constructed, and the salt-sensitive phenotype of this strain was complemented by introduction of a plasmid carrying the wild-type htrA gene, demonstrating that htrA is necessary for optimal growth under conditions of osmotic stress. Additionally, strain LDW1 was tested for its response to temperature and H(2)O(2) stresses. The results of these growth assays indicated that strain LDW1 grew at a lower rate than the wild-type strain at 44 degrees C but at a rate similar to that of the wild-type strain when incubated at 4 degrees C. In addition, strain LDW1 was significantly more sensitive to a 52 degrees C heat shock than the wild-type strain. Strain LDW1 was also defective in its response to H(2)O(2) challenge at 37 degrees C, since 100 or 150 micro g of H(2)O(2) was more inhibitory for the growth of strain LDW1 than for that of the parent strain. The stress response phenotype observed for strain LDW1 is similar to that observed for other HtrA(-) organisms, which suggests that L. monocytogenes HtrA may play a role in degrading misfolded proteins that accumulate under stress conditions.
Figures
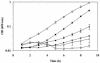
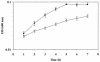
FIG. 1.
Effects of NaCl on growth of L. monocytogenes strains 10403S and OSM1. Strains 10403S and OSM1 were grown at 37°C in Pine's defined broth with varying concentrations of NaCl. Growth was measured by optical density at 600 nm. Growth curves represent the average optical density from two or more individual growth assays. Error bars, standard deviations among the growth assays. Solid symbols, 10403S; open symbols, OSM1. Squares, 1.8% NaCl; triangles, 3% NaCl; circles, 4% NaCl.
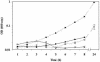
FIG. 2.
Effects of glycine betaine and NaCl on growth of L. monocytogenes strains 10403S and OSM1. Strains were grown at 37°C in Pine's defined broth with 4% NaCl in either the presence or the absence of 1 mM glycine betaine. Growth was measured by optical density at 600 nm. Growth curves represent the average optical density from two or more individual growth assays. Error bars, standard deviations among the growth assays. Symbols: ▪, 10403S; □, OSM1. Dashed lines represent cultures where 1 mM glycine betaine was added to the growth medium.
FIG. 3.
Location and arrangement of the htrA gene on the chromosomes of L. monocytogenes strains EGD-e and F2365 and L. innocua strain CLIP 11262. lmo0289, lmo0290, lmo0291, and lmo0293 correspond to the ORF names obtained from the annotated genome sequence of strain EGD-e. Horizontal arrows show the 5′-to-3′ orientation of each ORF. Solid rectangles, predicted ribosomal binding sites. The vertical arrow indicates the position of the predicted transcriptional terminator sequence for the htrA gene. The ranges of nucleotide distances between the htrA gene and the adjacent ORFs represent the distances obtained from the completed genome sequences of L. monocytogenes strains EGD-e and F2365 and L. innocua strain CLIP 11262.
FIG. 4.
Alignment of the L. monocytogenes (Lm) HtrA protein with HtrA homologs from B. subtilis (Bs), L. lactis (Ll), L. helveticus (Lh), Y. enterocolitica (Ye), and E. coli (Ec). Symbols below residues represent amino acid identity (*) and strong similarity (:). Arrows indicate the H-D-S catalytic residues. Boxed region, putative PDZ domain.
FIG. 5.
Growth of strain LDW1 in BHI with 5% added NaCl and complementation of the salt-sensitive phenotype with plasmid pLW1. Growth curves represent the average optical density from two or more individual growth assays. Error bars, standard deviations among the growth assays. (a) Growth of strains 10403S and LDW1 at 30°C in BHI broth with or without 5% added NaCl. Symbols: ▪, 10403S in BHI; □, LDW1 in BHI; ▴, 10403S in BHI plus 5% added NaCl; ▵, LDW1 in BHI plus 5% added NaCl. (b) Complementation of the NaCl-sensitive phenotype with a plasmid carrying the wild-type htrA gene. All strains were grown at 30°C in BHI broth plus chloramphenicol and 5% added NaCl. Symbols: ▪, LDW2 (10403S plus pCON-1); □, LDW4 (LDW1 plus pCON-1); ▴, LDW3 (10403S plus pLW1); ▵, LDW5 (LDW1 plus pLW1).
FIG. 6.
Effect of elevated temperature on growth of L. monocytogenes strains 10403S and LDW1. Cultures were grown in BHI with aeration at 44°C, and optical densities at 600 nm were measured hourly. Growth curves represent the average optical density from two or more individual growth assays. Error bars, standard deviations among the growth assays. Symbols: ▪, 10403S; □, LDW1.
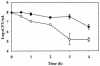
FIG. 7.
Effect of heat shock on survival of L. monocytogenes strains 10403S and LDW1. Cultures were grown at 30°C in BHI to mid-log phase and then shifted to 52°C. Samples were taken during the 4 h of heat shock and plated on BHI agar to enumerate survivors. Inactivation data were obtained from four individual cultures for each strain, and each experiment was run in duplicate. Error bars, standard deviations. Symbols: ▪, 10403S; □, LDW1.
Similar articles
- Listeria monocytogenes 10403S HtrA is necessary for resistance to cellular stress and virulence.
Wilson RL, Brown LL, Kirkwood-Watts D, Warren TK, Lund SA, King DS, Jones KF, Hruby DE. Wilson RL, et al. Infect Immun. 2006 Jan;74(1):765-8. doi: 10.1128/IAI.74.1.765-768.2006. Infect Immun. 2006. PMID: 16369036 Free PMC article. - Loss of SigB in Listeria monocytogenes Strains EGD-e and 10403S Confers Hyperresistance to Hydrogen Peroxide in Stationary Phase under Aerobic Conditions.
Boura M, Keating C, Royet K, Paudyal R, O'Donoghue B, O'Byrne CP, Karatzas KAG. Boura M, et al. Appl Environ Microbiol. 2016 Jul 15;82(15):4584-4591. doi: 10.1128/AEM.00709-16. Print 2016 Aug 1. Appl Environ Microbiol. 2016. PMID: 27208116 Free PMC article. - Role for serine protease HtrA (DegP) of Streptococcus pyogenes in the biogenesis of virulence factors SpeB and the hemolysin streptolysin S.
Lyon WR, Caparon MG. Lyon WR, et al. Infect Immun. 2004 Mar;72(3):1618-25. doi: 10.1128/IAI.72.3.1618-1625.2004. Infect Immun. 2004. PMID: 14977969 Free PMC article. - Role for HtrA in stress induction and virulence potential in Listeria monocytogenes.
Stack HM, Sleator RD, Bowers M, Hill C, Gahan CG. Stack HM, et al. Appl Environ Microbiol. 2005 Aug;71(8):4241-7. doi: 10.1128/AEM.71.8.4241-4247.2005. Appl Environ Microbiol. 2005. PMID: 16085809 Free PMC article. - The HtrA family of serine proteases.
Pallen MJ, Wren BW. Pallen MJ, et al. Mol Microbiol. 1997 Oct;26(2):209-21. doi: 10.1046/j.1365-2958.1997.5601928.x. Mol Microbiol. 1997. PMID: 9383148 Review.
Cited by
- Intracellular gene expression profile of Listeria monocytogenes.
Chatterjee SS, Hossain H, Otten S, Kuenne C, Kuchmina K, Machata S, Domann E, Chakraborty T, Hain T. Chatterjee SS, et al. Infect Immun. 2006 Feb;74(2):1323-38. doi: 10.1128/IAI.74.2.1323-1338.2006. Infect Immun. 2006. PMID: 16428782 Free PMC article. - Chaperone activity of serine protease HtrA of Helicobacter pylori as a crucial survival factor under stress conditions.
Zarzecka U, Harrer A, Zawilak-Pawlik A, Skorko-Glonek J, Backert S. Zarzecka U, et al. Cell Commun Signal. 2019 Dec 3;17(1):161. doi: 10.1186/s12964-019-0481-9. Cell Commun Signal. 2019. PMID: 31796064 Free PMC article. - Listeria monocytogenes PrsA2 is required for virulence factor secretion and bacterial viability within the host cell cytosol.
Alonzo F 3rd, Freitag NE. Alonzo F 3rd, et al. Infect Immun. 2010 Nov;78(11):4944-57. doi: 10.1128/IAI.00532-10. Epub 2010 Sep 7. Infect Immun. 2010. PMID: 20823208 Free PMC article. - Transcriptomic and phenotypic responses of Listeria monocytogenes strains possessing different growth efficiencies under acidic conditions.
Bowman JP, Lee Chang KJ, Pinfold T, Ross T. Bowman JP, et al. Appl Environ Microbiol. 2010 Jul;76(14):4836-50. doi: 10.1128/AEM.00315-10. Epub 2010 May 28. Appl Environ Microbiol. 2010. PMID: 20511423 Free PMC article. - Listeria monocytogenes mutants with altered growth phenotypes at refrigeration temperature and high salt concentrations.
Burall LS, Laksanalamai P, Datta AR. Burall LS, et al. Appl Environ Microbiol. 2012 Feb;78(4):1265-72. doi: 10.1128/AEM.06576-11. Epub 2011 Dec 16. Appl Environ Microbiol. 2012. PMID: 22179239 Free PMC article.
References
- Bayles, D. O., and B. J. Wilkinson. 2000. Osmoprotectants and cryoprotectants for Listeria monocytogenes. Lett. Appl. Microbiol. 30:23-27. - PubMed
- Begley, M., C. Hill, and C. G. M. Gahan. 2003. Identification and disruption of btlA, a locus involved in bile tolerance and general stress resistance in Listeria monocytogenes. FEMS Microbiol. Lett. 218:31-38. - PubMed
- Bianchi, A. A., and F. Baneyx. 1999. Hyperosmotic shock induces the σ32 and σE stress regulons of Escherichia coli. Mol. Microbiol. 34:1029-1038. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials