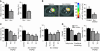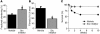Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction - PubMed (original) (raw)
. 2004 Mar;113(6):885-94.
doi: 10.1172/JCI20702.
Satoshi Shintani, Alberto Weber, Rudolf Kirchmair, Malcolm Wood, Adrianna Cravens, Heather McSharry, Atsushi Iwakura, Young-Sup Yoon, Nathan Himes, Deborah Burstein, John Doukas, Richard Soll, Douglas Losordo, David Cheresh
Affiliations
- PMID: 15067321
- PMCID: PMC362122
- DOI: 10.1172/JCI20702
Src blockade stabilizes a Flk/cadherin complex, reducing edema and tissue injury following myocardial infarction
Sara Weis et al. J Clin Invest. 2004 Mar.
Abstract
Ischemia resulting from myocardial infarction (MI) promotes VEGF expression, leading to vascular permeability (VP) and edema, a process that we show here contributes to tissue injury throughout the ventricle. This permeability/edema can be assessed noninvasively by MRI and can be observed at the ultrastructural level as gaps between adjacent endothelial cells. Many of these gaps contain activated platelets adhering to exposed basement membrane, reducing vessel patency. Following MI, genetic or pharmacological blockade of Src preserves endothelial cell barrier function, suppressing VP and infarct volume, providing long-term improvement in cardiac function, fibrosis, and survival. To our surprise, an intravascular injection of VEGF into healthy animals, but not those deficient in Src, induced similar endothelial gaps, VP, platelet plugs, and some myocyte damage. Mechanistically, we show that quiescent blood vessels contain a complex involving Flk, VE-cadherin, and beta-catenin that is transiently disrupted by VEGF injection. Blockade of Src prevents disassociation of this complex with the same kinetics with which it prevents VEGF-mediated VP/edema. These findings define a molecular mechanism to account for the Src requirement in VEGF-mediated permeability and provide a basis for Src inhibition as a therapeutic option for patients with acute MI.
Figures
Figure 1
Src blockade protects following myocardial infarction. (A) pp60Src–/– mice have significantly reduced myocardial water content and infarct size 24 hours after MI. (B) MRI T2 maps overlaid on gradient echo images in rats treated with vehicle or the PP1 Src inhibitor. Scale at right indicates T2 values from red (lower T2) to blue (higher T2). T2 values greater than 40 milliseconds were used as an index of edema, and representative images reveal reduced volume containing T2 values greater than 40 milliseconds 24 hours following MI in PP1-treated rats. Graph shows significant differences of the percentage of LVs with T2 values greater than 40 milliseconds between vehicle- and SKI-606 treated rats. (C) Treatment with a Src inhibitor results in significant and dose-dependent decreases in myocardial water content and infarct size after MI. Single-dose treatment with a Src inhibitor was optimally effective in reducing infarct size when administered 45 minutes after LAD ligation and still reduced infarct size significantly when administered up to 6 hours after infarct. (D) Src inhibition reduces infarct size and preserves function following transient ischemia and reperfusion. All panels represent the Src inhibitor PP1, except for B, as noted, and D, right panel, in which the Src inhibitor SKI-606 was used. *P < 0.05; **P < 0.001.
Figure 2
Effects of Src blockade on the long-term consequences of MI. Src inhibition delivered once 45 minutes after occlusion provides long-term protection even after 4–10 weeks. (A) Echocardiographic assessment reveals left ventricular function (% Fractional shortening) is improved after 4 weeks in rats that received the Src inhibitor. (B) Histological examination reveals less fibrosis (% LV area) at 4 weeks after occlusion in rats treated with the Src inhibitor. (C) Src inhibition improves survival of 2-year-old C57 black mice, a model that typically shows more than 40% mortality by 10 weeks. *P < 0.05.
Figure 3
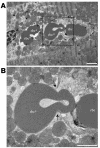
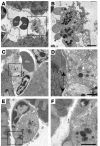
Ultrastructural changes to cardiac microvessels following MI or VEGF injection in mice. (A) Normal ventricular myocardium observed in a low-power transmission electron micrograph cross-section of cardiac myocytes and blood vessels. Scale bar: 20 μm. (B) Section transverse to myocytes showing normal myofilament architecture and mitochondria. Scale bar: 2 μm. (C) An rbc in the lumen of a normal microvessel with intact interendothelial junctions and consistent thickness of the endothelial layer. Scale bar: 1 μm. (D–I) Ultrastructural damage to blood vessels and ventricular myocardium from the peri-infarct region following MI or from left ventricular tissue following systemic VEGF injection. Images are representative, taken from either group. Summary of results appears in Table 1. (D) An rbc in the extracellular space adjacent to an abnormal blood vessel. Scale bar: 2 μm. “rbc” indicates red blood cell inside blood vessel; “rbc*” indicates red blood cell in extracellular space. (E) Enlargement of blood vessel in D, showing impaired interaction (arrows) between a swollen, electron-lucent EC and a neighboring EC. Scale bar: 1 μm. (F) Swollen, electron-lucent EC appears to restrict passage of rbc’s through vessel lumen. Scale bar: 1 μm. (G) Vessel with no apparent gaps, but three large vacuoles apparent in endothelium. Scale bar: 1 μm. (H) Severely affected myocyte (left) in peri-infarct zone with disintegrating myofilaments and mitochondria. Adjacent myocyte (right) appears less damaged. Scale bar: 2 μm. (I) Neutrophil (N) in blood vessel near myocyte damage. Scale bar: 5 μm.
Figure 4
Vascular leak at the ultrastructural level. (A) Microvessel containing one rbc and another rbc (rbc*) that is in the process of extravasation (boxed). Two other extravasated rbc’s (rbc*) are to the left. Note severe edema and disruption of myofilaments. Scale bar: 2 μm. (B) Enlargement of the boxed region in A showing extravasation site. Arrows indicate gap between ECs. Scale bar: 2 μm.
Figure 5
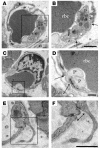
Platelet adhesion in blood vessels following MI or VEGF injection. (A and B) Microvessel containing an rbc with gap (arrows) between adjacent ECs (one of which appears electron lucent) in which two platelets (P) interact with intact basal lamina. Scale bar: 500 nm. (C and D) Gap in endothelial barrier (arrows) plugged by two degranulated platelets. Scale bar: 500 nm. (E and F) Platelet appears to interact with ECs to plug gap (arrow). Boxed regions in A, C, and E correspond with enlargements in B, D, and F, respectively. Scale bar: 500 nm.
Figure 6
Platelet aggregates impair blood flow through occluded vessels. (A–F) Microvessels containing rbc’s and platelet aggregates that protrude narrow extensions through (A and B) or interact with (C–F) discontinuous endothelium (arrows). P*, platelets appearing to directly contact basement membrane. Some platelets appear to be degranulated and/or have a less rounded shape, consistent with platelet activation. The platelet aggregates appear as five to eight platelets in plane of section and impede flow through part (A and B), most (C and D), or all (E and F) of the vessel lumen. Scale bars: 1 μm (B) and 500 nm (D–F).
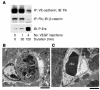
Figure 7
Biochemical signaling in vivo following VEGF is rapid and transient. (A–C) Immunoprecipitation (IP) and immunoblotting (IB) reveal a preformed Flk-cadherin-catenin complex that becomes phosphorylated and dissociates upon systemic VEGF stimulation in mice. (D) Src is required for these VEGF-mediated signaling events, as the Flk-cadherin-catenin complex remains intact in mice pretreated with the Src inhibitor PP1 before VEGF injection. (E) PP1 blocks VEGF-induced Src activity in a dose-dependent way, as assessed by phosphorylation of Src on Y418 and the Src substrate FAK on Y861. Each time point in this figure represents an individual mouse injected with VEGF and sacrificed after a particular duration, although the data shown are representative of at least three separate experiments. Because the events we measure appear to be rapid and transient, we do observe some variation between individual experiments, but we consistently measure phosphorylation events within 2–5 minutes that decrease after 15–30 minutes.
Figure 8
Multiple VEGF injections produce a persistent permeability response. (A) Signaling events following a single VEGF injection are transient and return to baseline by 30 minutes, while prolonged VEGF exposure over 2 hours yields a lasting signaling response. The Flk-cadherin and Flk-catenin complexes remain dissociated after prolonged VEGF exposure, and Erk phosphorylation is sustained. (B) Repeated VEGF injections over 2 hours elicited vascular remodeling similar to that seen in cardiac tissue 24 hours following MI. Microvessel with neutrophil and swollen electron-lucent EC (right side). (C) Vessel with swollen ECs partially occluding lumen. Scale bars: 2 μm.
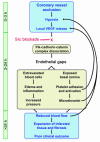
Figure 9
Sequence of cellular and molecular events following MI. The link between edema and poor clinical outcome following myocardial infarction has remained poorly understood. We propose a pathway in which hypoxia-driven VEGF expression following MI leads to edema and cardiac damage. Upon VEGF stimulation, Src activity is required for the disruption of a preformed Flk-cadherin-catenin complex, which loosens EC-cell contacts and permits extravasation of serum and blood cells. The resulting edema and inflammation increase interstitial pressure, which reduces local blood flow and causes further hypoxia. Endothelial gaps also expose basal lamina, attracting platelets that adhere, become activated, and could release VEGF locally. Platelet aggregates form microthrombi that can limit blood flow through smaller vessels, thereby increasing hypoxia and contributing to the expansion of cardiac damage and infarcted tissue that is associated with poor clinical outcome. Src blockade delivered within the first few hours following vessel occlusion could prevent VEGF-induced dissociation of the Flk-cadherin-catenin complex and preserve endothelial barrier integrity, thereby eliminating the further damage and infarct expansion beyond the initial ischemic boundary.
Similar articles
- Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis.
Weis S, Cui J, Barnes L, Cheresh D. Weis S, et al. J Cell Biol. 2004 Oct 25;167(2):223-9. doi: 10.1083/jcb.200408130. J Cell Biol. 2004. PMID: 15504909 Free PMC article. - Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke.
Paul R, Zhang ZG, Eliceiri BP, Jiang Q, Boccia AD, Zhang RL, Chopp M, Cheresh DA. Paul R, et al. Nat Med. 2001 Feb;7(2):222-7. doi: 10.1038/84675. Nat Med. 2001. PMID: 11175854 - Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site.
Wallez Y, Cand F, Cruzalegui F, Wernstedt C, Souchelnytskyi S, Vilgrain I, Huber P. Wallez Y, et al. Oncogene. 2007 Feb 15;26(7):1067-77. doi: 10.1038/sj.onc.1209855. Epub 2006 Aug 14. Oncogene. 2007. PMID: 16909109 - Angiogenic signal triggered by ischemic stress induces myocardial repair in rat during chronic infarction.
Fukuda S, Kaga S, Sasaki H, Zhan L, Zhu L, Otani H, Kalfin R, Das DK, Maulik N. Fukuda S, et al. J Mol Cell Cardiol. 2004 Apr;36(4):547-59. doi: 10.1016/j.yjmcc.2004.02.002. J Mol Cell Cardiol. 2004. PMID: 15081314 - Differential regulation of blood-brain barrier permeability in brain trauma and pneumococcal meningitis-role of Src kinases.
Paul R, Angele B, Popp B, Klein M, Riedel E, Pfister HW, Koedel U. Paul R, et al. Exp Neurol. 2007 Jan;203(1):158-67. doi: 10.1016/j.expneurol.2006.08.003. Epub 2006 Sep 28. Exp Neurol. 2007. PMID: 17010340
Cited by
- Adrenomedullin blockade induces regression of tumor neovessels through interference with vascular endothelial-cadherin signalling.
Khalfaoui-Bendriss G, Dussault N, Fernandez-Sauze S, Berenguer-Daizé C, Sigaud R, Delfino C, Cayol M, Metellus P, Chinot O, Mabrouk K, Martin PM, Ouafik L. Khalfaoui-Bendriss G, et al. Oncotarget. 2015 Apr 10;6(10):7536-53. doi: 10.18632/oncotarget.3167. Oncotarget. 2015. PMID: 25924235 Free PMC article. - Coronin 1B Controls Endothelial Actin Dynamics at Cell-Cell Junctions and Is Required for Endothelial Network Assembly.
Werner AC, Weckbach LT, Salvermoser M, Pitter B, Cao J, Maier-Begandt D, Forné I, Schnittler HJ, Walzog B, Montanez E. Werner AC, et al. Front Cell Dev Biol. 2020 Jul 31;8:708. doi: 10.3389/fcell.2020.00708. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32850828 Free PMC article. - Characterization of early myocardial inflammation in ischemia-reperfusion injury.
Wu Q, Xu R, Zhang K, Sun R, Yang M, Li K, Liu H, Xue Y, Xu H, Guo Y. Wu Q, et al. Front Immunol. 2023 Feb 6;13:1081719. doi: 10.3389/fimmu.2022.1081719. eCollection 2022. Front Immunol. 2023. PMID: 36814859 Free PMC article. - Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis.
Weis S, Cui J, Barnes L, Cheresh D. Weis S, et al. J Cell Biol. 2004 Oct 25;167(2):223-9. doi: 10.1083/jcb.200408130. J Cell Biol. 2004. PMID: 15504909 Free PMC article. - Neuronal Wiskott-Aldrich syndrome protein regulates Pseudomonas aeruginosa-induced lung vascular permeability through the modulation of actin cytoskeletal dynamics.
Che P, Wagener BM, Zhao X, Brandon AP, Evans CA, Cai GQ, Zhao R, Xu ZX, Han X, Pittet JF, Ding Q. Che P, et al. FASEB J. 2020 Feb;34(2):3305-3317. doi: 10.1096/fj.201902915R. Epub 2020 Jan 9. FASEB J. 2020. PMID: 31916311 Free PMC article.
References
- Garcia-Dorado D, Oliveras J. Myocardial oedema: a preventable cause of reperfusion injury? Cardiovasc. Res. 1993;27:1555–1563. - PubMed
- Senger DR, Perruzzi CA, Feder J, Dvorak HF. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer. Res. 1986;46:5629–5632. - PubMed
- Paul R, et al. Src deficiency or blockade of Src activity in mice provides cerebral protection following stroke. Nat. Med. 2001;7:222–227. - PubMed
- Eliceiri BP, et al. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol. Cell. 1999;4:915–924. - PubMed
- Breviario F, et al. Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin. Arterioscler. Thromb. Vasc. Biol. 1995;15:1229–1239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01-CA78045/CA/NCI NIH HHS/United States
- R37-CA50286/CA/NCI NIH HHS/United States
- R01 CA095262/CA/NCI NIH HHS/United States
- R01 HL057516/HL/NHLBI NIH HHS/United States
- HL-57516/HL/NHLBI NIH HHS/United States
- R37 CA050286/CA/NCI NIH HHS/United States
- HL-53354/HL/NHLBI NIH HHS/United States
- P01 HL066957/HL/NHLBI NIH HHS/United States
- R01 HL063414/HL/NHLBI NIH HHS/United States
- CA95262/CA/NCI NIH HHS/United States
- 1F32HL69701-02/HL/NHLBI NIH HHS/United States
- P01 CA078045/CA/NCI NIH HHS/United States
- HL-66957/HL/NHLBI NIH HHS/United States
- R37 HL053354/HL/NHLBI NIH HHS/United States
- R01 CA045726/CA/NCI NIH HHS/United States
- R24 EY014174/EY/NEI NIH HHS/United States
- P01-EY14174/EY/NEI NIH HHS/United States
- HL63609/HL/NHLBI NIH HHS/United States
- P01-HL57900/HL/NHLBI NIH HHS/United States
- HL-63695/HL/NHLBI NIH HHS/United States
- R01 HL063695/HL/NHLBI NIH HHS/United States
- HL-60911/HL/NHLBI NIH HHS/United States
- F32 HL069701/HL/NHLBI NIH HHS/United States
- P50 HL063609/HL/NHLBI NIH HHS/United States
- P01 HL057900/HL/NHLBI NIH HHS/United States
- HL-63414/HL/NHLBI NIH HHS/United States
- CA45726/CA/NCI NIH HHS/United States
- AG-16332/AG/NIA NIH HHS/United States
- RR14792/RR/NCRR NIH HHS/United States
- R01 HL053354/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous