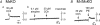M(3) muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice - PubMed (original) (raw)
M(3) muscarinic acetylcholine receptor plays a critical role in parasympathetic control of salivation in mice
Takeshi Nakamura et al. J Physiol. 2004.
Abstract
The M(1) and M(3) subtypes are the major muscarinic acetylcholine receptors in the salivary gland and M(3) is reported to be more abundant. However, despite initial reports of salivation abnormalities in M(3)-knockout (M(3)KO) mice, it is still unclear which subtype is functionally relevant in physiological salivation. In the present study, salivary secretory function was examined using mice lacking specific subtype(s) of muscarinic receptor. The carbachol-induced [Ca(2+)](i) increase was markedly impaired in submandibular gland cells from M(3)KO mice and completely absent in those from M(1)/M(3)KO mice. This demonstrates that M(3) and M(1) play major and minor roles, respectively, in the cholinergically induced [Ca(2+)](i) increase. Two-dimensional Ca(2+)-imaging analysis revealed the patchy distribution of M(1) in submandibular gland acini, in contrast to the ubiquitous distribution of M(3). In vivo administration of a high dose of pilocarpine (10 mg kg(-1), s.c.) to M(3)KO mice caused salivation comparable to that in wild-type mice, while no salivation was induced in M(1)/M(3)KO mice, indicating that salivation in M(3)KO mice is caused by an M(1)-mediated [Ca(2+)](i) increase. In contrast, a lower dose of pilocarpine (1 mg kg(-1), s.c.) failed to induce salivation in M(3)KO mice, but induced abundant salivation in wild-type mice, indicating that M(3)-mediated salivation has a lower threshold than M(1)-mediated salivation. In addition, M(3)KO mice, but not M(1)KO mice, had difficulty in eating dry food, as shown by frequent drinking during feeding, suggesting that salivation during eating is mediated by M(3) and that M(1) plays no practical role in it. These results show that the M(3) subtype is essential for parasympathetic control of salivation and a reasonable target for the drug treatment and gene therapy of xerostomia, including Sjögren's syndrome.
Figures
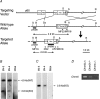
Figure 1. Generation of _Chrm5_-deficient mice
A, targeting strategy by homologous recombination in ES cells. The targeting vector pChrm5-N2 contained the neo gene and the diphtheria toxin α-subunit gene (DTA) driven by the phosphoglycerate kinase I promoter (PGK). The arrowheads marked MF16 and PR2 indicate the PCR primers used for homologous recombinant screening, and those marked MF23, AF2 and MR18 indicate the primers used for genotyping. _Apa_I (A), _Bam_HI (B), _Hin_dIII, _Eco_RI (R), _Sac_I (S), and _Ssp_I (Ss) sites relevant to the identification of homologous recombinant ES cell clones are shown, as well as the expected size of the bands hybridizing with the Chrm5 and neo probes (indicated by 4.9 and 2.8 kb bands and arrows). B and C, confirmation of homologous recombination in ES cell clones by Southern hybridization. B, hybridization with the Chrm5 probe showing the 2.8 kb band specific for the targeted allele and the 4.9 kb band derived from the wild-type allele. 5N-3 and 5N-4 are representative homologous recombinant clones, whereas RW4 is a parental ES cell line. C, hybridization with the neo probe showing the 2.8 kb band specific for the targeted allele. D, RT-PCR analysis showing Chrm5 mRNA levels in the brains of the wild-type and heterozygous and homozygous mutant mice. The levels were lower in the heterozygous brain than in the wild-type brain. No PCR product was seen with the homozygous mutant.
Figure 2. Carbachol (CCh)- and phenylephrine (PE)-induced [Ca2+]i changes in SMG cells from wild-type and KO mice
A_–_D, responses in individual representative mice. CCh or PE was applied to the SMG cells at the time point indicated by the arrow. The results shown are representative of 4 experiments. E_–_H, summarized peak [Ca2+]i (upper panel) and plateau [Ca2+]i (lower panel) increases induced by CCh (E_–_G; grey square: WT, black circle: KO) or PE (H; black column: WT, grey column: KO) in M1-, M3-, or M5-deficient mice (mean ±
s.d.
; n = 4 for each genotype). The [Ca2+]i was calculated using the equation described by Grynkiewicz et al. (1985). Control responses were measured in WT controls for each genotype.
Figure 3. CCh-induced [Ca2+]i increase in M3KO mice, its block by a muscarinic antagonist, atropine, and absence of the CCh-induced [Ca2+]i increase in M1/M3 double KO mice
A, SMG cells from M3KO mice; a, effect of CCh on the [Ca2+]I; b, block by atropine. The results shown in a and b were obtained using the same batch of SMG cells. B, effect of CCh or PE on the [Ca2+]i in SMG cells from M1/M3 double KO mice. Note the absence of CCh-induced [Ca2+]i increase. All the results shown are representative of 4 experiments.
Figure 4. Effect of thapsigargin (TG) and induction of capacitative Ca2+ entry (A_–_C), and Ca2+ release from the internal Ca2+ store by ionomycin (D and E) in WT, M3KO and M1/M3 double KO SMG cells
Drugs were applied to the SMG cell suspension at the time point indicated by the arrow. A_–_C, [Ca2+]i change in the nominal absence of external Ca2+ in response to 0.3 μ
m
thapsigargin (black trace) or control dimethylsulfoxide (DMSO; grey trace) and that caused by addition of 2 m
m
Ca2+. Note the large [Ca2+]i increase induced in all of the WT (A), M3KO (B) and M1/M3 double KO (C) SMG cells by adding external Ca2+ following TG application (capacitative Ca2+ entry). D, [Ca2+]i change in the absence of external Ca2+ in response to 10 μ
m
ionomycin in the WT, M3KO and M1/M3 double KO SMG cells. 1 m
m
EGTA was added to the external solution. E, summarized peak [Ca2+]i increases induced by 10 μ
m
ionomycin in WT, M3KO, or M1/M3 double KO SMG cells (mean ±
s.e.m.
; n = 4 for each genotype). All the results shown are representative of 4 experiments.
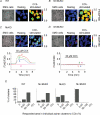
Figure 5. Ca2+ imaging in individual SMG acinar cell clusters and effect of CCh
A_–_D, image of SMG acinar cell clusters and pseudo-colour images of _F_340/_F_380 under resting and CCh-stimulated (30 μ
m
) conditions are shown (A, WT; B, M1/M5 double KO; C, M3KO; D, M1/M3 double KO SMG). Scale bars indicate 100 μm. Lower panels in C and D show the [Ca2+]i changes at regions 1–4 depicted in the upper panels. The results shown in A_–_D represent at least 8 separate measurements for each genotype. E, summarized responses of individual acinar cell clusters induced by 30 μ
m
CCh in WT, M1/M5 double KO, M3KO, or M1/M3 double KO SMG acinar cell clusters. Proportion of clusters categorized into three groups based on the responding area (0, 0–50 and 50–100%) as a precentage of the total number of clusters for each genotype was shown.
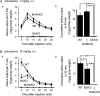
Figure 6. Cholinergically stimulated salivation in WT, M1/M5KO, M3KO and M1/M3KO mice
The saliva output in each 5 min period after stimulation is represented by the symbols and lines in the left panel (a) and the cumulative amount in 30 min in the right panel (b). A, salivation in response to 1 mg kg−1 of pilocarpine (
s.c.
) in WT (n = 5), M1/M5 double KO (n = 4) and M3KO (n = 5) mice. B, salivation in response to 10 mg kg−1 of pilocarpine (
s.c.
) in WT (n = 5), M3KO (n = 5), and M1/M3KO (n = 4) mice. The results are presented as the mean ±
s.e.m.
; some of the error bars are hidden by symbols. Statistical analysis was made using Scheffe's multiple comparisons following one-way ANOVA. In Aa, the values at all time points in M3KO mice showed a statistically significant difference (P < 0.01), compared to WT or M1/M5 double KO mice. In Ba, significantly smaller values in M3KO mice are depicted by asterisks (*P < 0.05, compared to WT), while the values at all time points in M1/M3 KO mice show statistically significant difference (P < 0.01, Student's t test), compared to WT or M3KO mice. In b, significantly smaller values were depicted by asterisks (*P < 0.05 and **P < 0.01).
Figure 7. Prandial drinking behaviour during food intake
Approaches to the water nozzle in 120 min during eating were measured in WT (n = 14), M1KO (n = 7), M1/M5KO (n = 11) and M3KO (n = 14) mice for dry food (A), or in WT (n = 5) and M3KO (n = 5) mice for hydrated food (B) (mean ±
s.e.m.
; *P < 0.01, Scheffe's multiple comparisons following one-way ANOVA).
Similar articles
- Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice.
Gautam D, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. Gautam D, et al. Mol Pharmacol. 2004 Aug;66(2):260-7. doi: 10.1124/mol.66.2.260. Mol Pharmacol. 2004. PMID: 15266016 - Cholinergically stimulated gastric acid secretion is mediated by M(3) and M(5) but not M(1) muscarinic acetylcholine receptors in mice.
Aihara T, Nakamura Y, Taketo MM, Matsui M, Okabe S. Aihara T, et al. Am J Physiol Gastrointest Liver Physiol. 2005 Jun;288(6):G1199-207. doi: 10.1152/ajpgi.00514.2004. Epub 2005 Feb 3. Am J Physiol Gastrointest Liver Physiol. 2005. PMID: 15691866 - Roles of M2 and M3 muscarinic receptors in cholinergic nerve-induced contractions in mouse ileum studied with receptor knockout mice.
Unno T, Matsuyama H, Izumi Y, Yamada M, Wess J, Komori S. Unno T, et al. Br J Pharmacol. 2006 Dec;149(8):1022-30. doi: 10.1038/sj.bjp.0706955. Epub 2006 Nov 13. Br J Pharmacol. 2006. PMID: 17099717 Free PMC article. - Autonomic control of salivary secretion.
Ekström J. Ekström J. Proc Finn Dent Soc. 1989;85(4-5):323-31; discussion 361-3. Proc Finn Dent Soc. 1989. PMID: 2699762 Review. - Muscarinic receptor agonists and antagonists: effects on ocular function.
Mitchelson F. Mitchelson F. Handb Exp Pharmacol. 2012;(208):263-98. doi: 10.1007/978-3-642-23274-9_12. Handb Exp Pharmacol. 2012. PMID: 22222703 Review.
Cited by
- The Regulatory Role of Rolipram on Inflammatory Mediators and Cholinergic/Adrenergic Stimulation-Induced Signals in Isolated Primary Mouse Submandibular Gland Cells.
Lee DU, Shin DM, Hong JH. Lee DU, et al. Mediators Inflamm. 2016;2016:3745961. doi: 10.1155/2016/3745961. Epub 2016 Apr 7. Mediators Inflamm. 2016. PMID: 27143817 Free PMC article. - Polarization of calcium signaling and fluid secretion in salivary gland cells.
Ambudkar IS. Ambudkar IS. Curr Med Chem. 2012;19(34):5774-81. doi: 10.2174/092986712804143321. Curr Med Chem. 2012. PMID: 23061636 Free PMC article. Review. - Botulinum toxin A inhibits salivary secretion of rabbit submandibular gland.
Shan XF, Xu H, Cai ZG, Wu LL, Yu GY. Shan XF, et al. Int J Oral Sci. 2013 Dec;5(4):217-23. doi: 10.1038/ijos.2013.82. Epub 2013 Oct 25. Int J Oral Sci. 2013. PMID: 24158141 Free PMC article. - Molecular cues for development and regeneration of salivary glands.
Liu F, Wang S. Liu F, et al. Histol Histopathol. 2014 Mar;29(3):305-12. doi: 10.14670/HH-29.305. Epub 2013 Nov 5. Histol Histopathol. 2014. PMID: 24189993 Free PMC article. Review. - Xerostomia and Its Cellular Targets.
Kim YJ. Kim YJ. Int J Mol Sci. 2023 Mar 10;24(6):5358. doi: 10.3390/ijms24065358. Int J Mol Sci. 2023. PMID: 36982432 Free PMC article. Review.
References
- Baum BJ. Principles of saliva secretion. Ann NY Acad Sci. 1993;694:17–23. - PubMed
- Baum BJ, Wellner RB. Receptors in salivary glands. In: Garett JR, Ekström J, Anderson LC, editors. Neural Mechanisms of Salivary Gland Secretion. Karger, Basel: 1999. pp. 44–58.
- Bell SM, Schreiner CM, Schultheis PJ, Miller ML, Evans RL, Vorhees CV, Shull GE, Scott WJ. Targeted disruption of the murine Nhe1 locus induces ataxia, growth retardation, and seizures. Am J Physiol. 1999;276:C788–C795. - PubMed
- Blazsek J, Varga G. Secretion from minor salivary glands following ablation of the major salivary glands in rats. Arch Oral Biol. 1999:S45–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous