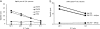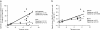Expansion of melanoma-specific cytolytic CD8+ T cell precursors in patients with metastatic melanoma vaccinated with CD34+ progenitor-derived dendritic cells - PubMed (original) (raw)
Expansion of melanoma-specific cytolytic CD8+ T cell precursors in patients with metastatic melanoma vaccinated with CD34+ progenitor-derived dendritic cells
Sophie Paczesny et al. J Exp Med. 2004.
Abstract
Cancer vaccines aim at inducing (a) tumor-specific effector T cells able to reduce/eliminate the tumor mass, and (b) long-lasting tumor-specific memory T cells able to control tumor relapse. We have shown earlier, in 18 human histocompatibility leukocyte antigen (HLA)-A*0201 patients with metastatic melanoma, that vaccination with peptide-loaded CD34-dendritic cells (DCs) leads to expansion of melanoma-specific interferon gamma-producing CD8+ T cells in the blood. Here, we show in 9 out of 12 analyzed patients the expansion of cytolytic CD8+ T cell precursors specific for melanoma differentiation antigens. These precursors yield, upon single restimulation with melanoma peptide-pulsed DCs, cytotoxic T lymphocytes (CTLs) able to kill melanoma cells. Melanoma-specific CTLs can be grown in vitro and can be detected in three assays: (a) melanoma tetramer binding, (b) killing of melanoma peptide-pulsed T2 cells, and (c) killing of HLA-A*0201 melanoma cells. The cytolytic activity of expanded CTLs correlates with the frequency of melanoma tetramer binding CD8+ T cells. Thus, CD34-DC vaccines can expand melanoma-specific CTL precursors that can kill melanoma antigen-expressing targets. These results justify the design of larger follow-up studies to assess the immunological and clinical response to peptide-pulsed CD34-DC vaccines.
Figures
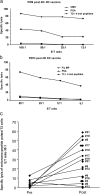
Figure 1.
CTL activity against melanoma peptide–pulsed T2 cells before and after vaccination. Purified CD8+ T cells after a single stimulation with peptide-pulsed DCs are used as effectors in a standard 4-h 51Cr assay with T2 cells pulsed with either a control PSA peptide, or with viral peptides (Flu-MP, CMV), or with a mix of the four melanoma peptides (GP100, MART-1/Melan A, tyrosinase, and MAGE-3) at indicated E/T ratios. (a) CD8+ T cells from patient number 9 (see Table I, after fourth DC vaccine) are capable of specific lysis (ordinate) of T2 cells pulsed with viral (CMV) or melanoma peptides at several E/T ratios (abscissa). (b) CD8+ T cells from patient number 13 (see Table I, after fourth DC vaccine) are capable of specific lysis (ordinate) of T2 cells pulsed with Flu-MP peptide at several E/T ratios (abscissa), but not of melanoma peptide–pulsed T2 cells. (c) Killing of melanoma peptide–pulsed T2 cells (a mix of four melanoma peptides) by cultured CD8+ T cells from all tested patients at baseline (Pre) and after fourth DC vaccination (Post). Ordinate/specific lysis at the E/T ratio of 30–25:1, after subtraction of values obtained with PSA peptide–loaded T2 (see Table S1), patient number (see Table I). All experiments are shown.
Figure 2.
CTL activity against allogenic tumor cell lines. Purified CD8+ T cells after single stimulation are used as effectors in a standard 4-h 51Cr assay with control (K562 and MCF-7) and HLA-A*0201 melanoma (Me275 and Me290) cell lines at the indicated E/T ratios. (a) Purified CD8+ T cells from patient number 12 (see Table I, after fourth DC vaccine) are capable of specific lysis (ordinate) of two melanoma cells lines, but do not kill control targets at several E/T ratios (abscissa). (b) Killing of Me275 cells is restricted by MHC class I expression on targets cells. Me275 cells are used without pretreatment or are preincubated with either an isotype control or anti–HLA class I W6/32 mAb and used as targets. Specific lysis (ordinate) at two E/T ratios (abscissa) representative of three independent experiments with T cells from three patients is shown.
Figure 3.
CTL activity against allogenic tumor cell lines before and after vaccination. Killing of Me275 melanoma cells, and of control MCF7 breast cancer and K562 cells, by cultured CD8+ T cells from all patients tested at baseline (Pre) and after fourth DC vaccine (Post). Ordinate, nonsubtracted specific lysis at the E/T ratio of 30–25:1; abscissa, patient number (see Table I). Average of all experiments for each patient (see Table S1) is shown. Prevaccination PBMCs were analyzed only once due to the limited availability of cells. Wilcoxon paired test.
Figure 4.
Flow cytometry analysis of tetramer binding by expanded CD8+ T cells. Restimulated CD8**+**T cells are labeled with anti–CD8-FITC (abscissa) and PE tetramers of a given specificity (ordinate). The analysis is performed on T cells gated on CD8 expression and the high affinity T cells are distinguished based on the intensity of tetramer fluorescence (square). (a) Patient number 17 and (b) patient number 21. For details, see Table S2. (c) Comparison of the percentage of high intensity tetramer binding CD8+ T cells (ordinate, log scale) at baseline and after fourth DC vaccination. Wilcoxon paired test.
Figure 5.
CTL function correlates with the frequency of tetramer-binding CD8+ T cells. (a) The specific lysis, by CD8+ T cells restimulated with melanoma peptide–pulsed DCs, of melanoma peptide–pulsed T2 cells (ordinate), but not of PSA peptide–pulsed T2 cells, correlates with the frequency of all high affinity/intensity melanoma tetramers binding CD8+ T cells determined used u-scores (abscissa). Nonparametric Spearman correlation. (b) The specific lysis of Me275 melanoma cells by CD8+T cells restimulated with melanoma peptide–pulsed DCs (ordinate) correlates with the frequency of all high affinity/intensity melanoma tetramers binding CD8+ T cells determined used u-scores (abscissa). There was no correlation between tetramer scores and the killing of control MCF7 cells. Nonparametric Spearman correlation.
Similar articles
- Novel approach to the characterization of melanoma associated-peptide-specific CTL lines from Japanese metastatic melanoma patients.
Akiyama Y, Maruyama K, Tai S, Takikawa M, Ohshita C, Yamamoto A, Yamazaki N, Kiyohara Y, Yamaguchi K. Akiyama Y, et al. Int J Oncol. 2008 Sep;33(3):433-41. Int J Oncol. 2008. PMID: 18695871 - Boosting vaccinations with peptide-pulsed CD34+ progenitor-derived dendritic cells can expand long-lived melanoma peptide-specific CD8+ T cells in patients with metastatic melanoma.
Palucka AK, Dhodapkar MV, Paczesny S, Ueno H, Fay J, Banchereau J. Palucka AK, et al. J Immunother. 2005 Mar-Apr;28(2):158-68. doi: 10.1097/01.cji.0000154249.74383.17. J Immunother. 2005. PMID: 15725960 - Novel dendritic cell-based vaccination in late stage melanoma.
Schneble EJ, Yu X, Wagner TE, Peoples GE. Schneble EJ, et al. Hum Vaccin Immunother. 2014;10(11):3132-8. doi: 10.4161/hv.29110. Hum Vaccin Immunother. 2014. PMID: 25483650 Free PMC article. Review. - Approaches to dendritic cell-based immunotherapy after peripheral blood stem cell transplantation.
Brugger W, Brossart P, Scheding S, Stuhler G, Heinrich K, Reichardt V, Grünebach F, Bühring HJ, Kanz L. Brugger W, et al. Ann N Y Acad Sci. 1999 Apr 30;872:363-71. doi: 10.1111/j.1749-6632.1999.tb08480.x. Ann N Y Acad Sci. 1999. PMID: 10372138 Review.
Cited by
- NAFLD indirectly impairs antigen-specific CD8+ T cell immunity against liver cancer in mice.
McVey JC, Green BL, Ruf B, McCallen JD, Wabitsch S, Subramanyam V, Diggs LP, Heinrich B, Greten TF, Ma C. McVey JC, et al. iScience. 2022 Feb 1;25(2):103847. doi: 10.1016/j.isci.2022.103847. eCollection 2022 Feb 18. iScience. 2022. PMID: 35198900 Free PMC article. - Harnessing the Complete Repertoire of Conventional Dendritic Cell Functions for Cancer Immunotherapy.
Amon L, Hatscher L, Heger L, Dudziak D, Lehmann CHK. Amon L, et al. Pharmaceutics. 2020 Jul 14;12(7):663. doi: 10.3390/pharmaceutics12070663. Pharmaceutics. 2020. PMID: 32674488 Free PMC article. Review. - Interleukin-33 pretreatment promotes metastatic growth of murine melanoma by reducing the cytotoxic capacity of CD8+ T cells and enhancing regulatory T cells.
Jevtovic A, Pantic J, Jovanovic I, Milovanovic M, Stanojevic I, Vojvodic D, Arsenijevic N, Lukic ML, Radosavljevic GD. Jevtovic A, et al. Cancer Immunol Immunother. 2020 Aug;69(8):1461-1475. doi: 10.1007/s00262-020-02522-x. Epub 2020 Apr 13. Cancer Immunol Immunother. 2020. PMID: 32285171 Free PMC article. - Combination Immunotherapy: Taking Cancer Vaccines to the Next Level.
Grenier JM, Yeung ST, Khanna KM. Grenier JM, et al. Front Immunol. 2018 Mar 22;9:610. doi: 10.3389/fimmu.2018.00610. eCollection 2018. Front Immunol. 2018. PMID: 29623082 Free PMC article. Review. - Dendritic Cell-Based Cancer Vaccines.
Santos PM, Butterfield LH. Santos PM, et al. J Immunol. 2018 Jan 15;200(2):443-449. doi: 10.4049/jimmunol.1701024. J Immunol. 2018. PMID: 29311386 Free PMC article. Review.
References
- Greenberg, P.D., J.P. Klarnet, D.E. Kern, and M.A. Cheever. 1988. Therapy of disseminated tumors by adoptive transfer of specifically immune T cells. Prog. Exp. Tumor Res. 32:104–127. - PubMed
- Herlyn, D., A. Linnenbach, H. Koprowski, and M. Herlyn. 1991. Epitope- and antigen-specific cancer vaccines. Int. Rev. Immunol. 7:245–257. - PubMed
- North, R.J. 1984. The murine antitumor immune response and its therapeutic manipulation. Adv. Immunol. 35:89–155. - PubMed
- Boon, T., J.C. Cerottini, B. Van den Eynde, P. van der Bruggen, and A. Van Pel. 1994. Tumor antigens recognized by T lymphocytes. Annu. Rev. Immunol. 12:337–365. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P0-1 CA84512/CA/NCI NIH HHS/United States
- RR00102/RR/NCRR NIH HHS/United States
- M01 RR000102/RR/NCRR NIH HHS/United States
- CA085540/CA/NCI NIH HHS/United States
- CA89440/CA/NCI NIH HHS/United States
- R01 CA085540/CA/NCI NIH HHS/United States
- CA78846/CA/NCI NIH HHS/United States
- P01 CA084512/CA/NCI NIH HHS/United States
- R01 CA089440/CA/NCI NIH HHS/United States
- R01 CA078846/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials