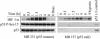Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide - PubMed (original) (raw)
Comparative Study
. 2004 Jun 15;101(24):8894-9.
doi: 10.1073/pnas.0400453101. Epub 2004 Jun 3.
Affiliations
- PMID: 15178764
- PMCID: PMC428443
- DOI: 10.1073/pnas.0400453101
Comparative Study
Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide
Douglas D Thomas et al. Proc Natl Acad Sci U S A. 2004.
Abstract
NO produced in tumors can either positively or negatively regulate growth. To examine this dichotomy, effects of NO concentration and duration on the posttranslational regulation of several key proteins were examined in human breast MCF7 cells under aerobic conditions. We found that different concentration thresholds of NO appear to elicit a discrete set of signal transduction pathways. At low steady-state concentrations of NO (<50 nM), extracellular signal-regulated kinase (ERK) phosphorylation was induced via a guanylate cyclase-dependent mechanism. Hypoxic inducible factor 1alpha (HIF-1alpha) accumulation was associated with an intermediate amount of NO (>100 nM), whereas p53 serine 15 phosphorylation occurred at considerably higher levels (>300 nM). ERK phosphorylation was transient during NO exposure. HIF-1alpha stabilization paralleled the presence of NO, whereas p53 serine 15 phosphorylation was detected during, and persisted after, NO exposure. The dose-dependent effects of synthetic NO donors were mimicked by activated macrophages cocultured with MCF7 cells at varying ratios. ERK and HIF-1alpha activation was similar in breast cancer cell lines either mutant (MB231) or null (MB157) in p53. The stabilization of HIF-1alpha by NO was not observed with increased MCF7 cell density, demonstrating the interrelationship between NO and O(2) consumption. The findings show that concentration and duration of NO exposure are critical determinants in the regulation of tumor-related proteins.
Figures
Fig. 1.
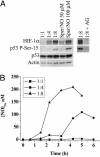
Temporal relationship between NO exposure and protein accumulation in MCF7 cells. Representative immunoblot of p53 P-Ser-15, HIF-1α, and pERK from MCF7 cell protein extracts after NO exposure (n > 3). Cells grown to 85% confluence in 150-mm Petri dishes were serum-starved overnight, treated with Sper/NO (100 μM), and harvested at the indicated time points. D, decomposed Sper/NO, 100 μM for 12 h; representative of all time points (data not shown).
Fig. 2.
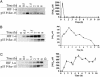
Protein accumulation as a function of NO concentration in MCF7 cells. (A) Cells grown to 85% confluence in 150-mm Petri dishes were serum-starved overnight and treated with Sper/NO as indicated. Time points were chosen corresponding to maximal protein accumulation (Fig. 1). D, decomposed Sper/NO (100 μM). (B) Cells were grown and treated with Sper/NO as in A. NO concentrations were determined from 100-μl sample aliquots of medium withdrawn from the Petri dish by gas-tight syringe without agitation and analyzed by chemiluminescence at the indicated time points. Representative data are shown as the mean ± SE (n = 3).
Fig. 3.
Comparison of HIF-1α and p53 P-Ser-15 accumulation by NO from either Sper/NO or activated macrophages. MCF7 cells were grown as in Fig. 1. (A) Four-hour treatment of MCF7 cells with either Sper/NO (50 and 100 μM) or ratios of activated NO-producing ANA-1 macrophages (MCF7:ANA-1) as indicated. 1:8 + AG = MCF7:ANA-1 1:8 + iNOS inhibitor aminoguanidine. (B) NO concentration from cocultured MCF7 and ANA-1 cells was determined as described in Fig. 2_B_. Representative data are shown (n = 3).
Fig. 4.
HIF-1α and p53 P-Ser-15 accumulation in response to various durations of NO exposure and real-time quantification of NO concentration. MCF7 cells were grown as in Fig. 1 and exposed to the NO donors. (A) 100 μM DEA/NO. (B) 100 μM Sper/NO. (C) 1,000 μM DETA/NO. NO steady-state levels were quantified as in Fig. 2_B_.
Fig. 5.
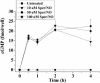
cGMP accumulation in MCF7 cells in response to various concentrations of the NO donor Sper/NO. MCF7 cells were grown to 50% confluency in a 96-well microtiter plates, serum-starved overnight, and treated with Sper/NO for the indicated time points (n = 3).
Fig. 6.
Effect of Hsp90 inhibition on NO-induced protein stabilization. MCF7 cells were grown as in Fig. 1. Cells were treated with Sper/NO (100 μM) ± geldanamycin (10 μM) and harvested at the indicated time points. HIF-1α and p53 P-Ser-15 were undetectable in untreated controls (data not shown).
Fig. 7.
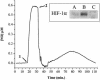
Effect of p53 status on NO-mediated HIF-1α stabilization. MB 231 and MB 157 breast cancer cells were grown as in Fig. 1, treated with Sper/NO (100 μM), and harvested at the indicated time points. MB 157 cells were grown in hypoxia for 4 h without Sper/NO as a HIF-1α positive control (4 hypoxia). A p53 P-Ser-15-positive MCF7 sample was blotted for an internal p53 control [p53 (+) control].
Fig. 8.
Electrochemical detection of NO in the presence of MCF7 cells. MCF7 cells were added in suspension (3 × 106/ml) as described in Materials and Methods. O2 was monitored continuously for 120 min (data not shown). Cells were isolated, and proteins were immunoblotted for HIF-1α. (A) Normoxia (≈21% O2); (B) hypoxia (<1% O2); (C) intermediate (≈10% O2). Representative NO electrode data for condition C are shown (n = 3). Sper/NO (100 μM) was added to the chamber (1). MCF7 cells were added after a steady-state NO level was achieved (2).
Similar articles
- Caffeine inhibits adenosine-induced accumulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and interleukin-8 expression in hypoxic human colon cancer cells.
Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Simioni C, Leung E, Maclennan S, Baraldi PG, Borea PA. Merighi S, et al. Mol Pharmacol. 2007 Aug;72(2):395-406. doi: 10.1124/mol.106.032920. Epub 2007 May 8. Mol Pharmacol. 2007. PMID: 17488804 - Impact of cyclic hypoxia on HIF-1alpha regulation in endothelial cells--new insights for anti-tumor treatments.
Martinive P, Defresne F, Quaghebeur E, Daneau G, Crokart N, Grégoire V, Gallez B, Dessy C, Feron O. Martinive P, et al. FEBS J. 2009 Jan;276(2):509-18. doi: 10.1111/j.1742-4658.2008.06798.x. Epub 2008 Dec 10. FEBS J. 2009. PMID: 19077164 - NO and transcriptional regulation: from signaling to death.
Zhou J, Brüne B. Zhou J, et al. Toxicology. 2005 Mar 15;208(2):223-33. doi: 10.1016/j.tox.2004.11.021. Toxicology. 2005. PMID: 15691587 Review. - [Redox-dependent regulation of gene expression induced by nitric oxide].
Turpaev KT, Litvinov DIu. Turpaev KT, et al. Mol Biol (Mosk). 2004 Jan-Feb;38(1):56-68. Mol Biol (Mosk). 2004. PMID: 15042836 Review. Russian.
Cited by
- NOS2 enhances KRAS-induced lung carcinogenesis, inflammation and microRNA-21 expression.
Okayama H, Saito M, Oue N, Weiss JM, Stauffer J, Takenoshita S, Wiltrout RH, Hussain SP, Harris CC. Okayama H, et al. Int J Cancer. 2013 Jan 1;132(1):9-18. doi: 10.1002/ijc.27644. Epub 2012 Jun 13. Int J Cancer. 2013. PMID: 22618808 Free PMC article. - Chemoprevention of Colon Cancer by iNOS-Selective Inhibitors.
Janakiram NB, Rao CV. Janakiram NB, et al. For Immunopathol Dis Therap. 2012 Jan 1;3(2):155-167. doi: 10.1615/ForumImmunDisTher.2012006186. For Immunopathol Dis Therap. 2012. PMID: 23678395 Free PMC article. - iNOS: a potential therapeutic target for malignant glioma.
Jahani-Asl A, Bonni A. Jahani-Asl A, et al. Curr Mol Med. 2013 Sep;13(8):1241-9. doi: 10.2174/1566524011313080002. Curr Mol Med. 2013. PMID: 23590833 Free PMC article. Review. - Long-term adaptation of breast tumor cell lines to high concentrations of nitric oxide.
Vesper BJ, Elseth KM, Tarjan G, Haines GK 3rd, Radosevich JA. Vesper BJ, et al. Tumour Biol. 2010 Aug;31(4):267-75. doi: 10.1007/s13277-010-0028-6. Epub 2010 May 18. Tumour Biol. 2010. PMID: 20480412 - Ischemic postconditioning attenuates liver warm ischemia-reperfusion injury through Akt-eNOS-NO-HIF pathway.
Guo JY, Yang T, Sun XG, Zhou NY, Li FS, Long D, Lin T, Li PY, Feng L. Guo JY, et al. J Biomed Sci. 2011 Oct 28;18(1):79. doi: 10.1186/1423-0127-18-79. J Biomed Sci. 2011. PMID: 22035453 Free PMC article.
References
- Juang, S. H., Xie, K., Xu, L., Shi, Q., Wang, Y., Yoneda, J. & Fidler, I. J. (1998) Hum. Gene. Ther. 9, 845–854. - PubMed
- Reveneau, S., Arnould, L., Jolimoy, G., Hilpert, S., Lejeune, P., Saint-Giorgio, V., Belichard, C. & Jeannin, J. F. (1999) Lab. Invest. 79, 1215–1225. - PubMed
- Scott, D. J., Hull, M. A., Cartwright, E. J., Lam, W. K., Tisbury, A., Poulsom, R., Markham, A. F., Bonifer, C. & Coletta, P. L. (2001) Gastroenterology 121, 889–899. - PubMed
- Wei, D., Richardson, E. L., Zhu, K., Wang, L., Le, X., He, Y., Huang, S. & Xie, K. (2003) Cancer Res. 63, 3855–3859. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous