Ubiquitin-dependent degradation of p73 is inhibited by PML - PubMed (original) (raw)
Ubiquitin-dependent degradation of p73 is inhibited by PML
Francesca Bernassola et al. J Exp Med. 2004.
Abstract
p73 has been identified recently as a structural and functional homologue of the tumor suppressor p53. Here, we report that p73 stability is directly regulated by the ubiquitin-proteasome pathway. Furthermore, we show that the promyelocytic leukemia (PML) protein modulates p73 half-life by inhibiting its degradation in a PML-nuclear body (NB)-dependent manner. p38 mitogen-activated protein kinase-mediated phosphorylation of p73 is required for p73 recruitment into the PML-NB and subsequent PML-dependent p73 stabilization. We find that p300-mediated acetylation of p73 protects it against ubiquitinylation and that PML regulates p73 stability by positively modulating its acetylation levels. As a result, PML potentiates p73 transcriptional and proapoptotic activities that are markedly impaired in Pml-/- primary cells. Our findings demonstrate that PML plays a crucial role in modulating p73 function, thus providing further insights on the molecular network for tumor suppression.
Figures
Figure 1.
p73 is degraded through the ubiquitin–proteasome pathway. (A) ts20 and ts41 cells were transfected with HA-p73α and either left at 34°C (lanes 1 and 3) or shifted to 40°C (lanes 2 and 4). Cell lysates were analyzed by immunoblot (IB) with anti-HA and anti–β-actin (top) antibodies. Expression of mRNA for p73 and β-actin was assessed by semi-quantitative RT-PCR analysis (bottom). (B) HCT-116(3) cells were either left untreated (lane 1) or incubated with 10 μM or lactacystin (lane 2) for 12 h. Cellular extracts were analyzed with anti-p73 antibody (clone 5B429). (C) Cos-1 cells were either transfected with the empty vector (lane 1) or with HA-Ub (lane 2) and treated with MG132 for 6 h. Cell lysates were immunoprecipitated with a rabbit polyclonal anti-p73 antibody followed by IB with anti-HA antibody. (D) ts20 cells grown at 34°C were transiently transfected with HA-p73α alone or in combination with a constitutively active form of MKK6. 8 h after transfection, some cultures were shifted to 40°C. (E) Phosphorylation of p73 in H1299 cells transfected with HA-p73α alone (lane 1) or in combination with HA-MKK6 (lane 2). Cells were cultured in [32P]orthophosphate-containing medium for 3 h, and phosphorylation was assessed by autoradiography. (F) In vitro phosphorylation of human purified His-p73α by p38 MAPK. 6 μg His-p73α was incubated with 1.2 U of recombinant purified p38 MAPK. ATF-2 was included as a control.
Figure 2.
PML protects p73 from ubiquitinylation and proteasome-mediated degradation. (A and B) PML increases the half-life of both exogenous and endogenous p73. p73 half-life was measured upon CHX (20 μg/ml) addition in H1299 cells transfected with either HA-p73α alone or with PML (A) and in wild type and Pml −/− MEFs (B). Cells lysates were examined by IB using anti-HA (A) or anti-p73 (B, clone 5B429) antibody. (C) H1299 cells were cotransfected with the indicated plasmids for 24 h. Some cultures were incubated with MG132 (lanes 5 and 6). p73-Ub immunocomplexes were analyzed by IB analysis with anti-HA (top) and anti-GFP (bottom) antibodies. (D) Cos-1 cells were either transfected with the empty vector (lane 1) or with Flag-PML (lane 2) for 24 h. Cell lysates were analyzed with rabbit polyclonal anti-p73, anti-Flag, and anti–β-actin antibody. Semi-quantitative RT-PCR analysis on p73 and β-actin mRNAs in Cos-1 cells was treated as aforementioned (bottom). (E) Cell lysates from wild type and Pml −/− MEFs were examined by IB using anti-p73 (clone 5B429) antibody. (F) Cos-1 cells were either left untreated (lane 1) or transfected with 200 pmol PML (lane 2) and Lamin A/C (lane 3) siRNA. After 48 h, cell lysates were prepared and analyzed for p73, PML, Lamin A/C, and β-actin expression by IB.
Figure 3.
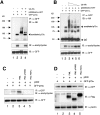
p73 and PML physically interact in vivo. (A) H1299 cells were transiently transfected with expression vectors encoding HA-p73α along with Flag-PML. p73–PML complexes were analyzed by IP using a monoclonal anti-PML (PG-M3) followed by IB with anti-HA and anti-Flag antibodies. Unspecific bands were recognized by the anti-Flag antibody in extracts from cells that did not overexpress PML (lanes 5 and 6). (B) Saos-2 cells inducible for p73α expression were transfected with Flag-PML. 4 h after transfection, cells were either left untreated or stimulated with 2 μg/ml doxycycline (Dox) for 24 h. Cell lysates were immunoprecipitated with anti-Flag antibody, and the immunoprecipitate was analyzed by IB using anti-HA antibody. (C) Schematic presentation of the structure of PML and its deletion mutants. (D) p73 interacts with the carboxy-terminal domain of PML in vivo (amino acids 436–633). Transformed 3T3 Pml −/− MEFs were cotransfected with GFP-p73α and full-length Flag-PML (lane 7), Flag-PML ΔRING (lane 8), Flag-PML RBCC (lane 9), or Flag-PML ΔXcmI. Cell lysates were immunoprecipitated with anti-Flag antibody and the immunoprecipitate analyzed by IB using anti-HA antibody. Inputs for both p73 and PML expression are shown. The ΔRING mutant comigrated with unspecific bands recognized by the anti-Flag antibody in the extracts (lanes 4 and 8).
Figure 4.
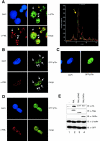
p73 and PML colocalization within the PML-NBs is required for p73 stabilization (A) Representative image of Cos-1 cells costained with anti-p73 (green, clone 5B429) and anti-PML (red) antibodies and analyzed by confocal microscopy (left). Nuclei were visualized by 4′,6-diamidino-2-phenylindole (DAPI) staining. The arrows indicate p73-containing speckles. (right) A quantitative analysis of the green and red fluorescence intensity at distinct nuclear speckles as indicated by the yellow arrow. (B–D) GFP-p73α was overexpressed into wild type (B) and Pml −/− (C) MEFs. Full-length PML was cotransfected with GFP-p73α into Pml −/− MEFs (D). Nuclei were visualized by DAPI staining. The arrows indicate p73-containing PML-NBs. (E) Pml −/− MEFs were transiently transfected with HA-p73α alone (lane 1) or in combination with full-length Flag-PML (lane 2), Flag-PML ΔRING (lane 3), or PML-RARα (lane 4). Cell extracts were subjected to IB with anti-HA, anti-Flag, anti-RARα, and anti–β-actin antibodies. (bottom) Normalization of transfection efficiency by quantitation of GFP expression.
Figure 5.
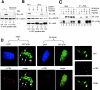
p38 MAPK is required for PML-mediated inhibition of p73 degradation. (A) ts20 cells were cotransfected with HA-p73α, GFP-spectrin with (lanes 2 and 4) or without (lanes 1 and 3) Flag-PML for 48 h at 34°C. Some cultures were treated with the p38 MAPK inhibitor SB202190 (lanes 3 and 4). (B) ts20 cells were cotransfected with GFP-spectrin, HA-p73α alone (lanes 1 and 2) or in combination with PML (lanes 4 and 5) in the absence (lane 4) or in the presence (lane 5) of p38(A/F). Whole cell extracts were immunoblotted with anti-HA, anti-p38, anti–β-actin, or anti-HSP90 antibodies. (C) Transfection and IP experiments were performed as described in Fig. 3 A. 8 h after transfection, cells were incubated with SB 202190 or DMSO for 20 h. (D) Wild-type MEFs were transfected with GFP-p73α and either left untreated or incubated with SB 202190 for 20 h. Cells were analyzed by confocal microscopy. Nuclei were visualized by DAPI staining. The arrows indicate p73-containing speckles. As a control, wild-type MEFs were also transfected with a GFP expression vector and stained with anti-PML (red) antibody (right).
Figure 6.
PML-regulated acetylation of p73 by p300 protects p73 against degradation. (A) GFP-p73α and HA-Ub were expressed in 293T cells in the absence (lane 3) or in the presence (lane 4) of p300bromo-HAT for 24 h. Cells were incubated with 1 μM trichostatin A (TSA) and 1 mM niacinamide for the last 12 h of transfection. After IP of GFP-p73, ubiquitinylation levels of p73 were detected with anti-HA antibody. Acetylated and unacetylated p73 in the immunoprecipitates were determined with anti–acetyl-lysine and anti-GFP antibody, respectively. (B) 293T cells were transfected with a plasmid encoding GFP-p73α alone (lane 1) or in combination with p300bromo-HAT (lanes 2–6), in the absence (lane 2) or in the presence (lanes 3–6) of decreasing doses of HA-Ub for 24 h. Treatment with deacetylase inhibitors and IP experiments were performed as described in A. (C and D) Transformed 3T3 Pml−/− MEFs were transiently transfected with GFP-p73α alone (lane 1) or along with p300 in the absence (lane 2) or in the presence of full-length Flag-PML (lane 3), Flag-PML-ΔRING (lane 4), or Flag-PML-RARα (lane 5) for 24 h. Cells were incubated with deacetylase inhibitors for 8 h. p73 was immunoprecipitated using anti-GFP antibody followed by IB with anti–acetyl-lysine antibody (C). Whole cell extracts were immunoblotted with anti-GFP, anti-Flag, and anti–β-actin antibodies (D).
Figure 7.
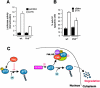
PML is required for p73 transcriptional and proapoptotic activity. (A) A bax promoter-driven luciferase reporter plasmid (bax-Luc) alone or in combination with p73α was transfected into wild type and Pml−/− MEFs, and luciferase activity was assayed 48 h after transfection. pRL-TK vector was included to normalize transfection efficiency, and reporter basal luciferase activity was normalized as 1. Values are mean ± SEM of two separate experiments each performed in duplicate. (B) Wild type and Pml−/− MEFs were retrovirally transduced with a pBabe-p73α construct for 48 h, selected in puromycin-containing medium for 3 d, and scored for apoptosis by FACS® analysis using propidium iodide staining. Values are mean ± SEM of two separate experiments each performed in duplicate. (C) A model for PML-mediated protection against p73 degradation. PML inhibits p73 ubiquitin-dependent degradation (left). PML controls p73 stability by regulating p73 acetylation levels in the PML-NB, thereby preventing its ubiquitinylation (right).
Similar articles
- Retinoic acid attenuates promyelocytic leukemia protein-induced cell death in breast cancer cells by activation of the ubiquitin-proteasome pathway.
Son SH, Yu E, Ahn Y, Choi EK, Lee H, Choi J. Son SH, et al. Cancer Lett. 2007 Mar 18;247(2):213-23. doi: 10.1016/j.canlet.2006.04.005. Epub 2006 Jun 5. Cancer Lett. 2007. PMID: 16740359 - A CK2-dependent mechanism for degradation of the PML tumor suppressor.
Scaglioni PP, Yung TM, Cai LF, Erdjument-Bromage H, Kaufman AJ, Singh B, Teruya-Feldstein J, Tempst P, Pandolfi PP. Scaglioni PP, et al. Cell. 2006 Jul 28;126(2):269-83. doi: 10.1016/j.cell.2006.05.041. Cell. 2006. PMID: 16873060 - PML, YAP, and p73 are components of a proapoptotic autoregulatory feedback loop.
Lapi E, Di Agostino S, Donzelli S, Gal H, Domany E, Rechavi G, Pandolfi PP, Givol D, Strano S, Lu X, Blandino G. Lapi E, et al. Mol Cell. 2008 Dec 26;32(6):803-14. doi: 10.1016/j.molcel.2008.11.019. Mol Cell. 2008. PMID: 19111660 - Regulation of the p73 protein stability and degradation.
Oberst A, Rossi M, Salomoni P, Pandolfi PP, Oren M, Melino G, Bernassola F. Oberst A, et al. Biochem Biophys Res Commun. 2005 Jun 10;331(3):707-12. doi: 10.1016/j.bbrc.2005.03.158. Biochem Biophys Res Commun. 2005. PMID: 15865926 Review. - p73 and p63 protein stability: the way to regulate function?
Maisse C, Guerrieri P, Melino G. Maisse C, et al. Biochem Pharmacol. 2003 Oct 15;66(8):1555-61. doi: 10.1016/s0006-2952(03)00511-2. Biochem Pharmacol. 2003. PMID: 14555234 Review.
Cited by
- On the Prevalence and Roles of Proteins Undergoing Liquid-Liquid Phase Separation in the Biogenesis of PML-Bodies.
Silonov SA, Mokin YI, Nedelyaev EM, Smirnov EY, Kuznetsova IM, Turoverov KK, Uversky VN, Fonin AV. Silonov SA, et al. Biomolecules. 2023 Dec 18;13(12):1805. doi: 10.3390/biom13121805. Biomolecules. 2023. PMID: 38136675 Free PMC article. - p63: a crucial player in epithelial stemness regulation.
Li Y, Giovannini S, Wang T, Fang J, Li P, Shao C, Wang Y; TOR centre; Shi Y, Candi E, Melino G, Bernassola F. Li Y, et al. Oncogene. 2023 Nov;42(46):3371-3384. doi: 10.1038/s41388-023-02859-4. Epub 2023 Oct 17. Oncogene. 2023. PMID: 37848625 Free PMC article. Review. - ZNF750: A Novel Prognostic Biomarker in Metastatic Prostate Cancer.
Montanaro M, Agostini M, Anemona L, Bonanno E, Servadei F, Finazzi Agrò E, Asimakopoulos AD, Ganini C, Cipriani C, Signoretti M, Bove P, Rugolo F, Imperiali B, Melino G, Mauriello A, Scimeca M. Montanaro M, et al. Int J Mol Sci. 2023 Mar 30;24(7):6519. doi: 10.3390/ijms24076519. Int J Mol Sci. 2023. PMID: 37047491 Free PMC article. - Control of protein stability by post-translational modifications.
Lee JM, Hammarén HM, Savitski MM, Baek SH. Lee JM, et al. Nat Commun. 2023 Jan 13;14(1):201. doi: 10.1038/s41467-023-35795-8. Nat Commun. 2023. PMID: 36639369 Free PMC article. Review. - Non-lysine ubiquitylation: Doing things differently.
Kelsall IR. Kelsall IR. Front Mol Biosci. 2022 Sep 19;9:1008175. doi: 10.3389/fmolb.2022.1008175. eCollection 2022. Front Mol Biosci. 2022. PMID: 36200073 Free PMC article. Review.
References
- Melino, G., V. De Laurenzi, and K.H. Vousden. 2002. p73: friend or foe in tumorigenesis. Nat. Rev. Cancer. 2:605–615. - PubMed
- Yang, A., M. Kaghad, D. Caput, and F. McKeon. 2002. On the shoulders of giants: p63, p73 and the rise of p53. Trends Genet. 18:90–95. - PubMed
- Jost, C.A., M.C. Marin, and W.G. Kaelin, Jr. 1997. p73 is a simian [correction of human] p53-related protein that can induce apoptosis. Nature. 389:191–194. - PubMed
- Kaghad, M., H. Bonnet, A. Yang, L. Creancier, J.C. Biscan, A. Valent, A. Minty, P. Chalon, J.M. Lelias, X. Dumont, et al. 1997. Monoallelically expressed gene related to p53 at 1p36, a region frequently deleted in neuroblastoma and other human cancers. Cell. 90:809–819. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-GM57587/GM/NIGMS NIH HHS/United States
- R01 GM057587/GM/NIGMS NIH HHS/United States
- R01 CA071692/CA/NCI NIH HHS/United States
- MC_U132670601/MRC_/Medical Research Council/United Kingdom
- R37 CA071692/CA/NCI NIH HHS/United States
- R01 CA076584/CA/NCI NIH HHS/United States
- CA-71692/CA/NCI NIH HHS/United States
- R01-CA76584/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous