The IL-6- and lipopolysaccharide-induced transcription of hepcidin in HFE-, transferrin receptor 2-, and beta 2-microglobulin-deficient hepatocytes - PubMed (original) (raw)
The IL-6- and lipopolysaccharide-induced transcription of hepcidin in HFE-, transferrin receptor 2-, and beta 2-microglobulin-deficient hepatocytes
Pauline Lee et al. Proc Natl Acad Sci U S A. 2004.
Abstract
The antimicrobial peptide hepcidin appears to play a central role in the regulation of iron homeostasis. In intact animals, iron overload or the injection of lipopolysaccharide (LPS) stimulates transcription of HAMP, the gene that encodes hepcidin. In isolated hepatocytes, IL-6, an inflammatory cytokine the production of which is stimulated by LPS, up-regulates transcription of hepcidin. In contrast, iron has no stimulatory effect on hepcidin expression in isolated hepatocytes. There is apparently a signaling pathway, activated by iron, that is present in the intact animal but not in isolated hepatocytes. Studies in humans and mice have shown that this iron-dependent pathway requires the presence of Hfe, hemojuvelin, and probably transferrin receptor 2 (tfr-2). To determine whether activation of hepcidin transcription by IL-6 also requires Hfe and tfr-2, we have studied mice homozygous for targeted disruption of HFE, beta(2)-microglobulin, and for a truncating mutation of TFR-2. We show that these mutant mice react normally to injection of endotoxin and that their isolated hepatocytes react normally to IL-6. This indicates that the signaling pathway activated by IL-6 does not require either Hfe or tfr-2. Mice with disruption of the gene encoding IL-6 seem to have a blunted response to LPS, but the statistical significance of the small response documented is borderline. It is therefore not clear whether LPS stimulates secretion of cytokines other than IL-6 that may stimulate hepcidin transcription.
Figures
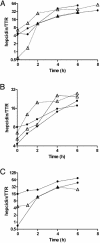
Fig. 1.
The response of isolated hepatocytes to treatment with IL-6. (A) The response of hepatocytes from HFE knockout mice. (B) tfr-2 mutant mice. (C)A β-microglobulin knockout mouse. In each, the response to IL-6 is compared with that of hepatocytes from the congenic strain also treated with IL-6. Open triangles represent the deficient mice; filled circles are the controls.
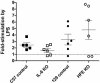
Fig. 2.
The hepcidin mRNA response of mice 6 h after i.p. injection of 1 μg of LPS per gram of body weight. Responses represent the ratio of hepcidin cDNA to TTR cDNA as measured by real-time PCR (see Materials and Methods). The results are presented as the percentage of the average hepcidin/TTR ratio documented in mice of the same age and strain that were injected with saline and killed after 6 h. Each point represents one mouse. The dashed line indicates the level at which there is no stimulation of hepcidin transcription by LPS. The mean and one standard error of the mean are shown.
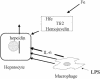
Fig. 3.
Schematic representation of the regulation of transcription of hepcidin by the iron pathway (above) and inflammatory (LPS/IL-6 pathway) (below). The iron pathway requires Hfe, hemojuvelin, and probably tfr-2 outside of the hepatocytes. These components are not required for the inflammatory pathway. Although IL-6 is an effective mediator of the response to LPS, some other mediators formed in response to LPS may also stimulate the transcription of hepcidin, although the evidence for this is unclear.
Similar articles
- Bone morphogenetic proteins 2, 4, and 9 stimulate murine hepcidin 1 expression independently of Hfe, transferrin receptor 2 (Tfr2), and IL-6.
Truksa J, Peng H, Lee P, Beutler E. Truksa J, et al. Proc Natl Acad Sci U S A. 2006 Jul 5;103(27):10289-10293. doi: 10.1073/pnas.0603124103. Epub 2006 Jun 26. Proc Natl Acad Sci U S A. 2006. PMID: 16801541 Free PMC article. - Transgenic HFE-dependent induction of hepcidin in mice does not require transferrin receptor-2.
Schmidt PJ, Fleming MD. Schmidt PJ, et al. Am J Hematol. 2012 Jun;87(6):588-95. doi: 10.1002/ajh.23173. Epub 2012 Mar 28. Am J Hematol. 2012. PMID: 22460705 Free PMC article. - The role of Hfe in transferrin-bound iron uptake by hepatocytes.
Chua AC, Herbison CE, Drake SF, Graham RM, Olynyk JK, Trinder D. Chua AC, et al. Hepatology. 2008 May;47(5):1737-44. doi: 10.1002/hep.22180. Hepatology. 2008. PMID: 18393371 - [HFE hemochromatosis: pathogenic and diagnostic approach].
Brissot P, Le Lan C, Troadec MB, Lorho R, Ropert M, Lescoat G, Loréal O. Brissot P, et al. Transfus Clin Biol. 2005 Jun;12(2):77-82. doi: 10.1016/j.tracli.2005.04.040. Transfus Clin Biol. 2005. PMID: 15925529 Review. French. - Non-HFE hemochromatosis.
Pietrangelo A. Pietrangelo A. Semin Liver Dis. 2005 Nov;25(4):450-60. doi: 10.1055/s-2005-923316. Semin Liver Dis. 2005. PMID: 16315138 Review.
Cited by
- Delivery of chemotherapeutic drugs in tumour cell-derived microparticles.
Tang K, Zhang Y, Zhang H, Xu P, Liu J, Ma J, Lv M, Li D, Katirai F, Shen GX, Zhang G, Feng ZH, Ye D, Huang B. Tang K, et al. Nat Commun. 2012;3:1282. doi: 10.1038/ncomms2282. Nat Commun. 2012. PMID: 23250412 - Normal iron metabolism and the pathophysiology of iron overload disorders.
Siah CW, Ombiga J, Adams LA, Trinder D, Olynyk JK. Siah CW, et al. Clin Biochem Rev. 2006 Feb;27(1):5-16. Clin Biochem Rev. 2006. PMID: 16886043 Free PMC article. - Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload.
Niederkofler V, Salie R, Arber S. Niederkofler V, et al. J Clin Invest. 2005 Aug;115(8):2180-6. doi: 10.1172/JCI25683. J Clin Invest. 2005. PMID: 16075058 Free PMC article. - Intravenous iron therapy and the cardiovascular system: risks and benefits.
Del Vecchio L, Ekart R, Ferro CJ, Malyszko J, Mark PB, Ortiz A, Sarafidis P, Valdivielso JM, Mallamaci F; ERA-EDTA European Renal and Cardiovascular Medicine Working (EURECA-m) Group. Del Vecchio L, et al. Clin Kidney J. 2020 Nov 26;14(4):1067-1076. doi: 10.1093/ckj/sfaa212. eCollection 2021 Apr. Clin Kidney J. 2020. PMID: 34188903 Free PMC article. Review. - The human symbiont Bacteroides thetaiotaomicron promotes diet-induced obesity by regulating host lipid metabolism.
Cho SH, Cho YJ, Park JH. Cho SH, et al. J Microbiol. 2022 Jan;60(1):118-127. doi: 10.1007/s12275-022-1614-1. Epub 2021 Dec 29. J Microbiol. 2022. PMID: 34964947
References
- Pigeon, C., Ilyin, G., Courselaud, B., Leroyer, P., Turlin, B., Brissot, P. & Loreal, O. (2001) J. Biol. Chem. 276, 7811-7819. - PubMed
- Nemeth, E., Valore, E. V., Territo, M., Schiller, G., Lichtenstein, A. & Ganz, T. (2003) Blood 101, 2461-2463. - PubMed
- Gehrke, S. G., Kulaksiz, H., Herrmann, T., Riedel, H. D., Bents, K., Veltkamp, C. & Stremmel, W. (2003) Blood 102, 371-376. - PubMed
- Ahmad, K. A., Ahmann, J. R., Migas, M. C., Waheed, A., Britton, R. S., Bacon, B. R., Sly, W. S. & Fleming, R. E. (2002) Blood Cells Mol. Dis. 29, 361-366. - PubMed
- Frazer, D. M. & Anderson, G. J. (2003) Blood Cells Mol. Dis. 30, 288-297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials