Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology - PubMed (original) (raw)
Review
Functions of NF-kappaB1 and NF-kappaB2 in immune cell biology
Sören Beinke et al. Biochem J. 2004.
Abstract
Two members of the NF-kappaB (nuclear factor kappaB)/Rel transcription factor family, NF-kappaB1 and NF-kappaB2, are produced as precursor proteins, NF-kappaB1 p105 and NF-kappaB2 p100 respectively. These are proteolytically processed by the proteasome to produce the mature transcription factors NF-kappaB1 p50 and NF-kappaB2 p52. p105 and p100 are known to function additionally as IkappaBs (inhibitors of NF-kappaB), which retain associated NF-kappaB subunits in the cytoplasm of unstimulated cells. The present review focuses on the latest advances in research on the function of NF-kappaB1 and NF-kappaB2 in immune cells. NF-kappaB2 p100 processing has recently been shown to be stimulated by a subset of NF-kappaB inducers, including lymphotoxin-beta, B-cell activating factor and CD40 ligand, via a novel signalling pathway. This promotes the nuclear translocation of p52-containing NF-kappaB dimers, which regulate peripheral lymphoid organogenesis and B-lymphocyte differentiation. Increased p100 processing also contributes to the malignant phenotype of certain T- and B-cell lymphomas. NF-kappaB1 has a distinct function from NF-kappaB2, and is important in controlling lymphocyte and macrophage function in immune and inflammatory responses. In contrast with p100, p105 is constitutively processed to p50. However, after stimulation with agonists, such as tumour necrosis factor-alpha and lipopolysaccharide, p105 is completely degraded by the proteasome. This releases associated p50, which translocates into the nucleus to modulate target gene expression. p105 degradation also liberates the p105-associated MAP kinase (mitogen-activated protein kinase) kinase kinase TPL-2 (tumour progression locus-2), which can then activate the ERK (extracellular-signal-regulated kinase)/MAP kinase cascade. Thus, in addition to its role in NF-kappaB activation, p105 functions as a regulator of MAP kinase signalling.
Figures
Figure 1. Schematic representation of members of the Rel/NF-κB (A) and IκB (B) families of proteins
Rel/NF-κB proteins are characterized by their conserved N-terminal RHDs (blue) which mediate DNA binding, nuclear localization and subunit dimerization. IκB proteins each contain ankyrin repeat regions (red), which interact with RHDs of NF-κB subunits to prevent nuclear translocation. The numbers of amino acids in each protein are shown on the right. In (A), arrowheads point to the C-terminal residues of p50 and p52, which are produced by proteolytic processing of the C-terminal halves p105 and p100 respectively (grey shading). In (B) the positions of the IKK phosphorylation sites, which regulate the inducible proteolysis of IκB proteins, are shown. LZ, leucine zipper; TD, transactivation domain.
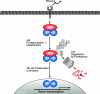
Figure 2. Model of the generic NF-κB activation pathway
Various stimuli induce the phosphorylation ( ) and subsequent polyubiquitination (
) and subsequent polyubiquitination ( ) of IκBs, which are then targeted for degradation by the 26 S proteasome. Associated NF-κB dimers are thereby released to translocate into the nucleus, where they bind to the promoter regions of NF-κB-responsive genes to modulate their expression. The transactivating capacity of nuclear NF-κB dimers can also be regulated by phosphorylation.
) of IκBs, which are then targeted for degradation by the 26 S proteasome. Associated NF-κB dimers are thereby released to translocate into the nucleus, where they bind to the promoter regions of NF-κB-responsive genes to modulate their expression. The transactivating capacity of nuclear NF-κB dimers can also be regulated by phosphorylation.
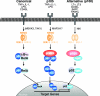
Figure 3. Model of different NF-κB signalling pathways
The canonical pathway (left) is activated by a large number of agonists, a few of which are listed. Activation of this pathway depends on the IKK complex (IKK1–IKK2–NEMO), which phosphorylates IκBα to induce its rapid degradation. (IκBβ and IκBε are similarly regulated by IKK.) This pathway is essential for immune responses, inflammation and promoting cell survival. The alternative NF-κB pathway (right) is activated a limited number of agonists and is important for secondary lymphoid organogenesis, maturation of B-cells and adaptive humoral immunity. This pathway requires NIK and IKK1 and induces the slow processing of p100 to p52, resulting in nuclear translocation of p52/RelB heterodimers. The p105 pathway (centre) is specifically involved in immune and inflammatory responses. Agonist activation of this pathway induces phosphorylation of the p105 PEST region by the classical IKK complex. This triggers p105 polyubiquitination and subsequent degradation, releasing p50 homodimers which undergo nuclear translocation and positively or negatively regulate gene transcription.
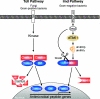
Figure 4. Drosophila Toll and Imd NF-κB signalling pathways
Stimulation of the Toll pathway by fungi and Gram-positive bacteria induces Cactus phosphorylation by an unknown kinase, triggering Cactus degradation by the proteasome. This releases Dif and Dorsal NF-κB-like transcription factor to translocate into the nucleus and induce the expression of antimicrobial peptides directed against Gram-positive bacteria and fungi. The Imd pathway is activated by Gram-negative bacteria, which trigger a cell-membrane-bound peptidoglycan recognition protein, PGRP-LC. Downstream of this receptor, the Imd signalling pathway bifurcates. One branch triggers the activation of the Drosophila melanogaster (dm) IKK complex, via the MAP 3-kinase dTAK1, which then directly phosphorylates Relish. The other branch regulates the activation of Dredd caspase. This directly cleaves phosphorylated Relish, generating the N-terminal transcription factor fragment (Rel-68) that translocates into the nucleus and induces the expression of antimicrobial peptides directed against Gram-negative bacteria. The IκB-like C-terminal fragment, Rel-49, remains in the cytoplasm, but does not prevent nuclear translocation of Rel-68. Imd is an adapter protein, containing a DD, which is required for activation of both the IKK and Dredd branches. ANK, ankyrin repeat region.
Figure 5. Proteolysis of NF-κB1 p105
NF-κB1 p50 is constitutively produced from p105 by proteolytic removal of the C-terminal half of p105 by the proteasome (a mechanism termed processing). The GRR acts as a stop signal, preventing entry of the RHD into the proteasome, which is thought to degrade p105 from the C-terminus. The stability of the folded RHD is important in preventing its entry into the proteasome and proteolysis. The identity of the E3 ligase involved in p105 processing is not known, but has been proposed to bind to an acidic region adjacent to the ankyrin repeats. Following agonist stimulation, p105 is phosphorylated by the IKK complex on Ser927 and Ser932 in the p105 PEST region. These phosphorylated residues act a binding site for βTrCP, the recognition subunit of a multisubunit SCF-type E3 ubiquitin ligase, which catalyses the polyubiquitination of phospho-p105 on multiple lysine residues, triggering its subsequent complete degradation by the proteasome. ROC1 is a RING finger protein. Elements involved in processing are shown in blue. Elements involved in degradation are shown in red.
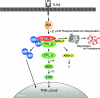
Figure 6. Diagram illustrating the TLR4–TPL-2–ERK–MAP kinase cascade
Stimulation of TLR4 by LPS activates the IKK complex which phosphorylates Ser927 and Ser932 in the p105 PEST region. This triggers p105 ubiquitination and subsequent degradation, liberating TPL-2. p105-free TPL-2 then phosphorylates and activates MEK, which in turn phosphorylates and activates ERK. In unstimulated cells, TPL-2 and p105 form a ternary complex with ABIN-2. ABIN-2 is released from p105 after LPS stimulation, but its function is currently unknown. IKK-induced p105 proteolysis also liberates associated p50 (and other Rel subunits) to translocate into the nucleus and modulate target gene expression.
Similar articles
- TPL-2 kinase regulates the proteolysis of the NF-kappaB-inhibitory protein NF-kappaB1 p105.
Belich MP, Salmerón A, Johnston LH, Ley SC. Belich MP, et al. Nature. 1999 Jan 28;397(6717):363-8. doi: 10.1038/16946. Nature. 1999. PMID: 9950430 - Nuclear factor-kappaB1: regulation and function.
Pereira SG, Oakley F. Pereira SG, et al. Int J Biochem Cell Biol. 2008;40(8):1425-30. doi: 10.1016/j.biocel.2007.05.004. Epub 2007 May 17. Int J Biochem Cell Biol. 2008. PMID: 17693123 Review. - Hepatitis B virus HBx protein activates transcription factor NF-kappaB by acting on multiple cytoplasmic inhibitors of rel-related proteins.
Su F, Schneider RJ. Su F, et al. J Virol. 1996 Jul;70(7):4558-66. doi: 10.1128/JVI.70.7.4558-4566.1996. J Virol. 1996. PMID: 8676482 Free PMC article. - Coordinate regulation of TPL-2 and NF-κB signaling in macrophages by NF-κB1 p105.
Yang HT, Papoutsopoulou S, Belich M, Brender C, Janzen J, Gantke T, Handley M, Ley SC. Yang HT, et al. Mol Cell Biol. 2012 Sep;32(17):3438-51. doi: 10.1128/MCB.00564-12. Epub 2012 Jun 25. Mol Cell Biol. 2012. PMID: 22733995 Free PMC article. - Regulation and function of NF-kappaB transcription factors in the immune system.
Vallabhapurapu S, Karin M. Vallabhapurapu S, et al. Annu Rev Immunol. 2009;27:693-733. doi: 10.1146/annurev.immunol.021908.132641. Annu Rev Immunol. 2009. PMID: 19302050 Review.
Cited by
- Gut instinct: harnessing the power of probiotics to tame pathogenic signaling pathways in ulcerative colitis.
Hsu CY, Mustafa MA, Moath Omar T, Taher SG, Ubaid M, Gilmanova NS, Nasrat Abdulraheem M, Saadh MJ, Athab AH, Mirzaei R, Karampoor S. Hsu CY, et al. Front Med (Lausanne). 2024 Sep 11;11:1396789. doi: 10.3389/fmed.2024.1396789. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39323474 Free PMC article. Review. - NF-κB2 mutation targets survival, proliferation and differentiation pathways in the pathogenesis of plasma cell tumors.
McCarthy BA, Yang L, Ding J, Ren M, King W, ElSalanty M, Zakhary I, Sharawy M, Cui H, Ding HF. McCarthy BA, et al. BMC Cancer. 2012 May 29;12:203. doi: 10.1186/1471-2407-12-203. BMC Cancer. 2012. PMID: 22642622 Free PMC article. - The immunostimulatory effect of indole-6-carboxaldehyde isolated from Sargassum thunbergii (Mertens) Kuntze in RAW 264.7 macrophages.
Park C, HwangBo H, Lee H, Kim GY, Cha HJ, Choi SH, Kim S, Kim HS, Yun SJ, Kim WJ, Jeon YJ, Choi YH. Park C, et al. Anim Cells Syst (Seoul). 2020 Aug 20;24(4):233-241. doi: 10.1080/19768354.2020.1808529. Anim Cells Syst (Seoul). 2020. PMID: 33029301 Free PMC article. - Double plant homeodomain (PHD) finger proteins DPF3a and -3b are required as transcriptional co-activators in SWI/SNF complex-dependent activation of NF-κB RelA/p50 heterodimer.
Ishizaka A, Mizutani T, Kobayashi K, Tando T, Sakurai K, Fujiwara T, Iba H. Ishizaka A, et al. J Biol Chem. 2012 Apr 6;287(15):11924-33. doi: 10.1074/jbc.M111.322792. Epub 2012 Feb 13. J Biol Chem. 2012. PMID: 22334708 Free PMC article. - NF-kappaB1 contributes to T cell-mediated control of Toxoplasma gondii in the CNS.
Harris TH, Wilson EH, Tait ED, Buckley M, Shapira S, Caamano J, Artis D, Hunter CA. Harris TH, et al. J Neuroimmunol. 2010 May;222(1-2):19-28. doi: 10.1016/j.jneuroim.2009.12.009. Epub 2010 Feb 13. J Neuroimmunol. 2010. PMID: 20156658 Free PMC article.
References
- Medzhitov R. Toll-like receptors and innate immunity. Nat. Rev. Immunol. 2001;1:135–145. - PubMed
- Takeda K., Kaisho T., Akira S. Toll-like receptors. Annu. Rev. Immunol. 2003;21:335–376. - PubMed
- O'Neill L. A. J., Dinarello C. A. The IL-1 receptor/Toll-like receptor superfamily: crucial receptors for inflammation and host defense. Immunol. Today. 2000;21:206–209. - PubMed
- Wallach D., Varfolomeev E. E., Malinin N. L., Goltsev Y. V., Kovalenko A. V., Boldin M. P. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 1999;17:331–367. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous