NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis - PubMed (original) (raw)
NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis
Debajit K Biswas et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Lack of molecular targets in estrogen receptor-negative (ER-negative) breast cancer is a major therapeutic hurdle. We studied NF-kappa B activation in human breast tumors and in carcinoma cell lines. Activated NF-kappa B was detected predominantly in ER-negative vs. ER-positive breast tumors and mostly in ER-negative and ErbB2-positive tumors (86%). These in vivo results demonstrate association of activated NF-kappa B with a subgroup of human breast tumors and are consistent with previously reported in vitro observations using similar classes of human breast cancer cell lines. Finding such an association suggested functional and biological significance. Immunofluorescence demonstrated increased nuclear p65, a component of the active NF-kappa B complex, in cytokeratin 19 (CK19)-positive epithelial cells of ER-negative/ErbB2-positive tumor samples. In contrast, nuclear NF-kappa B was detected mostly in stroma of ER-negative and ErbB2-negative tumors, suggesting a role of activated NF-kappa B in intercellular signaling between epithelial and stromal cells in this type of breast cancers. To elucidate roles of activated NF-kappa B, we used an ER-negative and ErbB2-positive human breast tumor cell line (SKBr3). The polypeptide heregulin beta1 stimulated, and herceptin, the anti-ErbB2 antibody, inhibited, NF-kappa B activation in SKBr3 cells. The NF-kappa B essential modulator (NEMO)-binding domain (NBD) peptide, an established selective inhibitor of I kappa B-kinase (IKK), blocked heregulin-mediated activation of NF-kappa B and cell proliferation, and simultaneously induced apoptosis only in proliferating and not resting cells. These results substantiate the hypothesis that certain breast cancer cells rely on NF-kappa B for aberrant cell proliferation and simultaneously avoid apoptosis, thus implicating activated NF-kappa B as a therapeutic target for distinctive subclasses of ER-negative breast cancers.
Figures
Fig. 1.
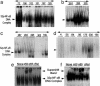
Detection of active NF-κB in extracts of human breast cancer tissues by EMSA. Tumor specimens are designated with numbers indicated in each panel (tissue bank numbers) and classified by their ER and ErbB2 receptor levels. Arrows to the left of each representative gel show the 32P–NF-κB–DNA complex. (a) EMSA-binding activity for six of the seven class 1 (ER-negative/ErbB2-positive) tumors. (b) Three tumors with positive binding results were chosen from nine tumors in class 2 (ER-negative/ErbB2-negative). (c and d) Five representative tumors from eight in class 3 (ER-positive/ErbB2-positive) are displayed (c) and seven of seven from class 4 (ER-positive/ErbB2-negative) are shown (d). Each panel displays duplicates for each tumor determination. (e and f) Supershifted 32P–NF-κB–DNA complexes. (e) Tumor BOT 72 from class 1. (f) Tumor BOT 288 from class 2, each shifted with three anti-rel protein antibodies (against p50, p65, and c-rel). The upper arrow shows the supershifted band.
Fig. 2.
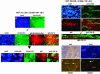
Localization of active NF-κB in frozen sections from human breast cancers. Localization of NF-κB complexes was performed in frozen sections from each EMSA-positive tumor (Fig. 1). (a_–_k) Sections from the ER-negative/ErbB2-positive and NF-κB-EMSA-positive sample BOT 312 were stained by double antibody immunofluorescence (23). (a) DAPI-stained nuclei in a representative section from BOT 312 at low power (×10). (b) The epithelial cell marker CK19 is demonstrated by green fluorescence and shown in the same section at the same magnification as in a. Higher power images of regions containing epithelial cells are shown at ×40 (c_–_f). A field of cells is shown stained with DAPI (c), with antibodies to CK19 (d), and with antibodies detecting p65 (e). Superimposed images by using
photoshop
software (Adobe Systems, San Jose, CA) demonstrate colocalization of nuclear p65 (red fluorescence) encircled by green fluorescence, generated by cytoplasmic CK19 in the same cell (f). These signals are further elaborated by digital enlargements of the superimposed CK19 and nuclear p65 containing epithelial cells from the boxed area of panels c_–_f (g_–_k). DAPI-stained nuclei (g) are superimposed with p65 staining to demonstrate the nuclear localization of this NF-κB component (h), and colocalization of cytoplasmic CK19 (i) and nuclear p65 (j) is demonstrated in merged images (k). Levels of CK19 and p65 in a similarly analyzed frozen section of the class 2 ER-negative/ErbB2-negative/NF-κB-positive tumor specimen, BOT 288, is shown (l_–_o). (l) The distribution of DAPI-stained nuclei shows the overall cellularity of the section at ×40. (m) CK19-stained epithelial cells show cords off cells infiltrating tumor. (n) p65-positive cells appear distinct from CK19-positive epithelial cells, and (o) the merged image shows that CK19- and p65-positive cells are in separate islands. (p) An enlarged presentation of panel o shows CK19 cells with vacant, unstained nuclei. (q_–_t) BOT 288 was also examined for the presence of the p50/p65 complex, studied by using mixtures of antibodies to p50 and p65 with double immunofluorescence. A low-power hematoxylin and eosin-stained section (q) and the corresponding DAPI-stained nuclei (r) show the border of the tumor pushing against the surrounding stroma. The p50 and p65 associated merged red and green fluorescence generated the brownish yellow signals in the nuclei of the cells. Few nuclei in the tumor stained for both components of active NF-κB(s); however, more frequent colocalization was seen in merged images of the stromal cells (t).
Fig. 3.
Modulation of heregulin-induced activation of NF-κB by herceptin and NBD peptide in SKBr3 cells. Nuclear extracts from SKBr3 cells were prepared and NF-κB–32P–DNA-binding activity was determined by EMSA. (a) Binding of 32P-labeled oligonucleotides to NF-κB at the indicated times after treatment of the cells with 1 nM heregulin is shown in duplicate (lanes 1–4). The identities of the NF-κB components were determined by supershift assays with antibodies to rel-family proteins p50 and p65 (data not shown). (b) NF-κB–32P–DNA-binding activity was inhibited by simultaneous treatment of SKBr3 cells with the indicated concentrations of heregulin and herceptin, measured at 18 h after application. (c) Heregulin-stimulated NF-κB–32P–DNA-binding activity in SKBr3 cells and simultaneous treatment with WT NBD (lanes 2–4) and mutant NBD (lanes 5–7). The DMSO concentration at the highest NBD concentration was 1%, and the control reaction (lane 1) contained the same amount of DMSO.
Fig. 4.
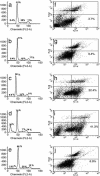
Cell-cycle progression and apoptosis in heregulin and NBD peptide-treated SKBr3 cells. Growth of cells and drug treatment conditions were the same as in Fig. 3. The fraction of cells in different phases of the cell cycle was measured by propidium iodide (PI) staining followed by FACS (Becton Dickinson) analysis. Cell-cycle distribution of cells grown in rich medium (a), in serum-free medium (b), in serum-free medium in the presence of heregulin (1 nM) (c), in serum-free medium in the presence of heregulin (1 nM) and WT NBD (100 μM) (d), and in serum-free medium in the presence of heregulin (1 nM) and mutant NBD (100 μM) (e). Numbers in each panel show the percent distribution of cycling cells (excluding the dead sub G0 population) in different phases under these treatment conditions. The apoptotic fraction of cells detected by annexin V staining after different treatments is shown, with numerals in the lower right-hand panel of each figure showing the annexin V-positive fraction (f_–_j). (f) Cells grown in serum-free medium in the presence of heregulin (1 nM). (g) Cells grown in serum-free medium in the presence of WT NBD (100 μM). (h and i) Cells grown in serum-free medium in the presence of heregulin (1 nM) and WT NBD at 50 μM (h) and 100 μM (i). (j) Treatment with the mutant NBD peptide at 100 M showed minimal or no effect. All treatments were for 18 h.
Fig. 5.
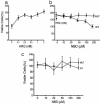
Influence of selective inhibition of NF-κB activation by NBD peptide on heregulin-induced cell proliferation. Duplicate cultures of cells were seeded in 96-well plates in rich medium. Twenty-four hours later, the rich medium was replaced with serum-free medium and treated with indicated agents, and cell numbers were determined by MTT assay after 72 h of treatment. Assays were normalized to cell counts without any treatments, taken as 100%. (a) SKBr3 cell proliferation in serum-free medium in the presence of the indicated concentrations of heregulin. (b) The same number of cells grown in the presence of heregulin (1 nM) and the indicated concentrations of WT or mutant (MUT) NBD peptide. (c) Cell proliferation in the presence of WT or mutant (MUT) NBD peptide in the absence of heregulin.
Similar articles
- Nuclear factor-kappaB activation: a molecular therapeutic target for estrogen receptor-negative and epidermal growth factor receptor family receptor-positive human breast cancer.
Singh S, Shi Q, Bailey ST, Palczewski MJ, Pardee AB, Iglehart JD, Biswas DK. Singh S, et al. Mol Cancer Ther. 2007 Jul;6(7):1973-82. doi: 10.1158/1535-7163.MCT-07-0063. Mol Cancer Ther. 2007. PMID: 17620428 - Nitric Oxide-Releasing Aspirin Suppresses NF-κB Signaling in Estrogen Receptor Negative Breast Cancer Cells in Vitro and in Vivo.
Nath N, Chattopadhyay M, Rodes DB, Nazarenko A, Kodela R, Kashfi K. Nath N, et al. Molecules. 2015 Jul 9;20(7):12481-99. doi: 10.3390/molecules200712481. Molecules. 2015. PMID: 26184135 Free PMC article. - Linkage between EGFR family receptors and nuclear factor kappaB (NF-kappaB) signaling in breast cancer.
Biswas DK, Iglehart JD. Biswas DK, et al. J Cell Physiol. 2006 Dec;209(3):645-52. doi: 10.1002/jcp.20785. J Cell Physiol. 2006. PMID: 17001676 Review. - Up-regulation of vascular endothelial growth factor C in breast cancer cells by heregulin-beta 1. A critical role of p38/nuclear factor-kappa B signaling pathway.
Tsai PW, Shiah SG, Lin MT, Wu CW, Kuo ML. Tsai PW, et al. J Biol Chem. 2003 Feb 21;278(8):5750-9. doi: 10.1074/jbc.M204863200. Epub 2002 Dec 5. J Biol Chem. 2003. PMID: 12471041 - Crossroads of estrogen receptor and NF-kappaB signaling.
Biswas DK, Singh S, Shi Q, Pardee AB, Iglehart JD. Biswas DK, et al. Sci STKE. 2005 Jun 14;2005(288):pe27. doi: 10.1126/stke.2882005pe27. Sci STKE. 2005. PMID: 15956359 Review.
Cited by
- Hypoxia and aging: molecular mechanisms, diseases, and therapeutic targets.
Nisar A, Khan S, Li W, Hu L, Samarawickrama PN, Gold NM, Zi M, Mehmood SA, Miao J, He Y. Nisar A, et al. MedComm (2020). 2024 Oct 15;5(11):e786. doi: 10.1002/mco2.786. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39415849 Free PMC article. Review. - Anti-Inflammatory and Cancer-Preventive Potential of Chamomile (Matricaria chamomilla L.): A Comprehensive In Silico and In Vitro Study.
Drif AI, Yücer R, Damiescu R, Ali NT, Abu Hagar TH, Avula B, Khan IA, Efferth T. Drif AI, et al. Biomedicines. 2024 Jul 5;12(7):1484. doi: 10.3390/biomedicines12071484. Biomedicines. 2024. PMID: 39062057 Free PMC article. - E3 ubiquitin ligase BCA2 promotes breast cancer stemness by up-regulation of SOX9 by LPS.
Zheng M, Liu W, Zhang R, Jiang D, Shi Y, Wu Y, Ge F, Chen C. Zheng M, et al. Int J Biol Sci. 2024 Apr 29;20(7):2686-2697. doi: 10.7150/ijbs.92338. eCollection 2024. Int J Biol Sci. 2024. PMID: 38725852 Free PMC article. - BMP7 alleviates trigeminal neuralgia by reducing oligodendrocyte apoptosis and demyelination.
Chen K, Wei X, Wang R, Yang L, Zou D, Wang Y. Chen K, et al. J Headache Pain. 2023 Oct 24;24(1):143. doi: 10.1186/s10194-023-01681-3. J Headache Pain. 2023. PMID: 37875834 Free PMC article. - Metabolic Regulation of Copper Toxicity during Marine Mussel Embryogenesis.
Young T, Gale SL, Ragg NLC, Sander SG, Burritt DJ, Benedict B, Le DV, Villas-Bôas SG, Alfaro AC. Young T, et al. Metabolites. 2023 Jul 11;13(7):838. doi: 10.3390/metabo13070838. Metabolites. 2023. PMID: 37512545 Free PMC article.
References
- Perou, C. M., Sorlie, T., Eisen, M. B., van de Rijn, M., Jeffrey, S. S., Rees, C. A., Pollack, J. R., Ross, D. T., Johnsen, H., Akslen, L. A., et al. (2000) Nature 406, 747-752. - PubMed
- Schnitt, S. J. & Guidi, A. J. (2000) in Diseases of the Breast, eds. Harris, J. R., Lippman, M. E., Morrow, M. & Osborne, C. K. (Lippincott Williams & Wilkins, Philadelphia), 2nd Ed., pp. 425-470.
- Menard, S., Tagliabue, E., Campiglio, M. & Pupa, S. M. (2000) J. Cell Physiol. 182, 150-162. - PubMed
- Elledge, R. M. & Fuqua S. A. W. (2000) in Diseases of the Breast, eds. Harris, J. R., Lippman, M. E., Morrow, M. & Osborne, C. K. (Lippincott Williams & Wilkins, Philadelphia), 2nd Ed., pp. 471-488.
- Yarden, Y. & Sliwkowski, M. X. (2001) Nat. Rev. Mol. Cell Biol. 2, 127-137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous