Protein and mRNA expression of uPAR and PAI-1 in myoepithelial cells of early breast cancer lesions and normal breast tissue - PubMed (original) (raw)
Comparative Study
Protein and mRNA expression of uPAR and PAI-1 in myoepithelial cells of early breast cancer lesions and normal breast tissue
R Hildenbrand et al. Br J Cancer. 2004.
Abstract
Myoepithelial cells (MEs), which surround ducts and acini of the breast glands, exhibit an anti-invasive phenotype and form a natural border separating proliferating tumour cells of ductal carcinoma in situ (DCIS) from basement membrane (bm) and underlying stroma. Invasion requires penetration of these host cellular and extracellular matrix barriers. This destruction is caused by proteolytic activity of tumour cells and host bystander cells. There is substantial evidence that high concentrations of the urokinase plasminogen-activating system are conducive to tumour cell spread and metastasis. Prompted by the conspicuous absence of studies examining the role of the ME in breast cancer progression, we studied the expression of the urokinase plasminogen activator receptor (uPAR) and plasminogen activator inhibitor type-1 (PAI-1) in MEs of 60 DCIS samples. Our results show that nearly all MEs of DCIS and normal breast glands exhibit the uPAR antigen, whereas the PAI-1 antigen was mainly expressed in MEs of high-grade DCIS. In one intermediate DCIS numerous ducts showed an incomplete myoepithelial layer expressing uPAR and PAI-1. We conclude that uPAR in MEs may be necessary to attach them to the bm by uPAR/vitronectin (Vn) interaction. The strong expression of PAI-1, which is known to resolve the uPAR/Vn binding, may be involved in the detachment of MEs of DCIS. Although the role of PAI-1 acting as cell detachment factor could not be demonstrated in our study, we speculate that the loss of the anti-invasive ME layer in DCIS may be triggered by PAI-1 and could be an early sign of subsequent tumour cell infiltration.
Figures
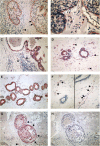
Figure 1
(A) Anti-uPAR HU277 immunoreaction of a high-grade DCIS. Myoepithelial cells show a strong staining (arrows) and tumour cells only a faint immunoreaction. Macrophages (arrowhead) are also positive. (B) Anti-uPAR IID7 immunoreaction of a high-grade DCIS. Myoepithelial cells show a strong immunoreaction (arrows) and tumour cells and endothelial cells are negative. Macrophages express the uPAR antigen (arrowhead). (C) Anti-PAI-1 immunoreaction of a high-grade DCIS. Tumour cells, endothelial cells (arrow) and stromal cells (arrowhead) are positive. The myoepithelial cell layer is absent; in corresponding tissue sections no MEs in this duct were observed using anti-calponin and anti-SMA antibodies. (D) Normal (nontumour) breast tissue stained for anti-uPAR IID7; MEs, epithelial cells and endothelial cells (arrow) show a strong immunoreaction, stromal cells (macrophages and fibroblasts) (arrowheads) are also positive. (E) Double staining of normal (nontumour) breast tissue stained for anti-uPAR IID7 (red colour) and anti-SMA (black colour); MEs show an immunoreaction for both anti-uPAR and anti-SMA. (F) Normal (nontumour) breast tissue stained for anti-PAI-1; in both images, the ducts (MEs and epithelial cells) are negative, whereas endothelial cells (arrows) and stromal cells (arrowheads) show positive immunoreactions. (G, H) Nonisotopic in situ hybridisation using fluorescein-labelled oligodeoxynucleotides complementary to PAI-1 mRNA in a non-high-grade DCIS with necrosis; (G) antisense probe: a distinct reaction in MEs (arrows) and tumour cells, stromal cells and endothelial cells (arrowhead) is observed; (H) no reaction is seen with the sense probe.
Figure 2
(A–D) Represent one intermediate-grade DCIS stained with mAb anti-uPA (A), anti-PAI-1 (B) and anti-uPAR IID7 (C, D). (A) Myoepithelial cells (arrows) show a positive anti-uPA immunoreaction, tumour cells show only a faint reaction; (B) same DCIS as in image B, showing an incomplete ME layer with a strong anti-PAI-1 immunoreaction; tumour cells show a faint immunoreaction. (C, D) Same DCIS as in images A and B stained with mAb anti-uPAR IID7; the ME layer in image C is absent and the tumour cells are partly detached from the bm; MEs in image D show a strong anti-uPAR immunoreaction; tumour cells in both images are weakly anti-uPAR positive; luminal macrophages (arrow) in image C strongly express the uPAR antigen. (E) Represents a high-grade DCIS double stained for anti-PAI-1 (red colour) and anti-SMA (black colour); MEs are positive for both anti-PAI-1 and anti-SMA, tumour cells, stromal cells (arrowhead) and endothelial cells (arrow) strongly express the PAI-1 antigen. (F) Represents a high-grade DCIS double stained for anti-uPAR IID7 (red colour) and anti-SMA (black colour); MEs are positive for both anti-uPAR and anti-SMA; tumour cells and stromal cells strongly express the uPAR antigen. (G, H) In situ hybridisation using fluorescein-labelled oligodeoxynucleotides complementary to uPAR-mRNA in a non-high-grade DCIS (grade 1); (G) antisense probe: a distinct reaction in MEs, tumour cells, stromal cells and endothelial cells is observed; (H) no reaction is seen with sense probe.
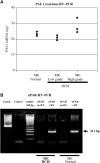
Figure 3
(A) Quantitative RT–PCR of mRNA derived from microdissected MEs of normal breast tissue, low- and high-grade DCIS using PAI-1-specific primers. From each group one case was selected and a LightCycler™ analysis was performed in triplicate. The amount of PAI-1 mRNA was calculated by assorting each CP to a standard curve. (B) The RNA of MEs (DCIS and normal breast tissue) was isolated, reverse transcriptase reaction followed by a PCR using uPAR primers (see Materials and methods) was performed. The RT–PCR reveals a 311 bp product in both probes (DCIS and normal breast tissue); without RT reaction no product was received.
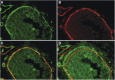
Figure 4
Immunofluorescence double staining of a high-grade DCIS with mAb anti-Vn ((A) red signal) and anti-collagen type-4 ((B) green signal); (C) both immunoreactions are associated within the bm of the breast duct and of a blood vessel; (D) DCIS double immunolabelled for Vn and collagen type-4 with a 10-pixel shift of the red signal (collagen type-4), revealing clearly a green and red signal in the bm's.
Similar articles
- The urokinase-system in tumor tissue stroma of the breast and breast cancer cell invasion.
Hildenbrand R, Schaaf A. Hildenbrand R, et al. Int J Oncol. 2009 Jan;34(1):15-23. Int J Oncol. 2009. PMID: 19082473 - Plasminogen activator system localization in 60 cases of ductal carcinoma in situ.
Hurd TC, Sait S, Kohga S, Winston J, Martinick M, Saxena R, Lankes H, Markus G, Harvey S, Gibbs JF. Hurd TC, et al. Ann Surg Oncol. 2007 Nov;14(11):3117-24. doi: 10.1245/s10434-007-9529-y. Epub 2007 Aug 16. Ann Surg Oncol. 2007. PMID: 17701256 - Combined overexpression of urokinase, urokinase receptor, and plasminogen activator inhibitor-1 is associated with breast cancer progression: an immunohistochemical comparison of normal, benign, and malignant breast tissues.
Costantini V, Sidoni A, Deveglia R, Cazzato OA, Bellezza G, Ferri I, Bucciarelli E, Nenci GG. Costantini V, et al. Cancer. 1996 Mar 15;77(6):1079-88. doi: 10.1002/(sici)1097-0142(19960315)77:6<1079::aid-cncr12>3.0.co;2-z. Cancer. 1996. PMID: 8635127 - [The clinical prospects for the study of the plasminogen activation system in breast cancer].
Gershteĭn ES, Kushlinskiĭ NE. Gershteĭn ES, et al. Vestn Ross Akad Med Nauk. 1999;(8):58-61. Vestn Ross Akad Med Nauk. 1999. PMID: 10487126 Review. Russian. - Structure, function and expression on blood and bone marrow cells of the urokinase-type plasminogen activator receptor, uPAR.
Plesner T, Behrendt N, Ploug M. Plesner T, et al. Stem Cells. 1997;15(6):398-408. doi: 10.1002/stem.150398. Stem Cells. 1997. PMID: 9402652 Review.
Cited by
- Deciphering the molecular basis of breast cancer metastasis with mouse models.
Vernon AE, Bakewell SJ, Chodosh LA. Vernon AE, et al. Rev Endocr Metab Disord. 2007 Sep;8(3):199-213. doi: 10.1007/s11154-007-9041-5. Rev Endocr Metab Disord. 2007. PMID: 17657606 Review. - Downregulation of the Ubiquitin-E3 Ligase RNF123 Promotes Upregulation of the NF-κB1 Target SerpinE1 in Aggressive Glioblastoma Tumors.
Wang X, Bustos MA, Zhang X, Ramos RI, Tan C, Iida Y, Chang SC, Salomon MP, Tran K, Gentry R, Kravtsova-Ivantsiv Y, Kelly DF, Mills GB, Ciechanover A, Mao Y, Hoon DSB. Wang X, et al. Cancers (Basel). 2020 Apr 27;12(5):1081. doi: 10.3390/cancers12051081. Cancers (Basel). 2020. PMID: 32349217 Free PMC article. - Microenvironmental regulation of cancer development.
Hu M, Polyak K. Hu M, et al. Curr Opin Genet Dev. 2008 Feb;18(1):27-34. doi: 10.1016/j.gde.2007.12.006. Epub 2008 Feb 20. Curr Opin Genet Dev. 2008. PMID: 18282701 Free PMC article. Review. - A Three-mRNA Signature Associated with Pyrimidine Metabolism for Prognosis of BRCA.
Zhang X, Zhang Q, Xie X, Li Y, Pang Z, Yu T. Zhang X, et al. Biomed Res Int. 2022 Feb 16;2022:7201963. doi: 10.1155/2022/7201963. eCollection 2022. Biomed Res Int. 2022. PMID: 35224098 Free PMC article. - Amplification of the urokinase-type plasminogen activator receptor (uPAR) gene in ductal pancreatic carcinomas identifies a clinically high-risk group.
Hildenbrand R, Niedergethmann M, Marx A, Belharazem D, Allgayer H, Schleger C, Ströbel P. Hildenbrand R, et al. Am J Pathol. 2009 Jun;174(6):2246-53. doi: 10.2353/ajpath.2009.080785. Epub 2009 May 12. Am J Pathol. 2009. PMID: 19435784 Free PMC article.
References
- Casey JR, Petranka JG, Kottra J, Fleenor DE, Rosse WF (1994) The structure of the urokinase-type plasminogen activator receptor-gene. Blood 84: 1151–1156 - PubMed
- Gebb C, Haymann EG, Engvall E, Ruoslahti E (1986) Interaction with vitronectin and collagen. J Biol Chem 261: 16698–16703 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous