Dopamine, through the extracellular signal-regulated kinase pathway, downregulates CD4+CD25+ regulatory T-cell activity: implications for neurodegeneration - PubMed (original) (raw)
Dopamine, through the extracellular signal-regulated kinase pathway, downregulates CD4+CD25+ regulatory T-cell activity: implications for neurodegeneration
Jonathan Kipnis et al. J Neurosci. 2004.
Abstract
Fighting off neuronal degeneration requires a well controlled T-cell response against self-antigens residing in sites of the CNS damage. The ability to evoke this response is normally suppressed by naturally occurring CD4+CD25+ regulatory T-cells (Treg). No physiological compound that controls Treg activity has yet been identified. Here, we show that dopamine, acting via type 1 dopamine receptors (found here to be preferentially expressed by Treg), reduces the suppressive activity and the adhesive and migratory abilities of Treg. Treg activity was correlated with activation of the ERK1/2 (extracellular signal-regulated kinase 1/2) signaling pathway. Systemic injection of dopamine or an agonist of its type 1 receptors significantly enhanced, via a T-cell-dependent mechanism, protection against neuronal death after CNS mechanical and biochemical injury. These findings shed light on the physiological mechanisms controlling Treg and might open the way to novel therapeutic strategies for downregulating Treg activity (e.g., in neuronal degeneration) or for strengthening it (in autoimmune diseases).
Figures
Figure 1.
Dopamine (DA) reduces the suppressive activity mediated by CD4+CD25+ regulatory T-cells. Proliferation of Teff (a CD4+CD25- population) was assayed by incorporation of [3H]-thymidine into Teff cocultured with naturally occurring Treg. Recorded values are from one of three representative experiments and are expressed as means ± SD of four replicates. a, Treg were activated by incubation for 24 hr with anti-CD3 antibodies in the presence of mrIL-2. Incubation of the activated Treg for 2 hr with dopamine (10-5 or 10-7
m
) before their coculturing with Teff reduced their suppression of Teff compared with that obtained with Treg not exposed to dopamine. b, Dopamine (10-5, 10-7, or 10-9
m
) added to freshly purified Treg. Dopamine (10-5 and 10-7
m
) had a similar effect on activity of naive Treg to that of to activated Treg, whereas the effect of dopamine at 10-9
m
on Treg-mediated suppression was not significant. c, Activation of Treg for 96 hr, followed by the addition of dopamine (10-5
m
) for 2 hr at the end of activation, significantly reduced the suppressive activity of Treg on Teff. Incubation of Teff with dopamine (10-5
m
) for 2 hr did not affect their susceptibility to Treg-induced suppression.
Figure 2.
Dopamine effect on Treg is mediated via D1-type receptor family. Proliferation of Teff (a CD4+CD25- population) was assayed by incorporation of [3H]-thymidine into Teff cocultured with naturally occurring Treg. Recorded values are from one of three representative experiments and are expressed as means ± SD of four replicates. a, Addition of norepinephrine (NE; 10-5 or 10-7
m
) to Treg for 2 hr after their activation for 24 hr did not affect the suppressive activity of Treg. Significant differences between groups were analyzed by Student's t test (p < 0.001). b, The inhibitory effect of dopamine (DA) on the suppressive activity of Treg was mimicked by SKF-38393, a specific agonist of the D1-type family. The D2-type agonist quinpirole did not alter the effect of dopamine on Treg. SCH 23390, a specific D1-type antagonist, wiped out the dopamine effect on the suppressive activity of Treg. Each experiment was performed at least five times, and representative results are shown. c, Incubation of Treg or Teff with dopamine did not cause apoptosis, as shown by propidium iodide staining for DNA content and FACS analysis of Treg and Teff, 48 hr after their incubation for 2 hr with dopamine. d, Staining for apoptosis with annexin V for phosphatidylserine on a surface membrane. No increase in annexin V-labeled cells was detected on incubation of Treg with dopamine or with the D1-type agonist SKF-38393.
Figure 3.
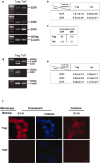
Preferential expression of D1-type receptors by Treg. Expression of dopamine receptors on Treg and Teff was investigated on mRNA and protein levels. a, b, Semiquantitative reverse transcription-PCR (RT-PCR) analysis for D1-R and D5-R expression. mRNA was extracted from freshly isolated Teff and Treg. The housekeeping gene β-actin was used for quantitative analysis. The results of one of five representative experiment are shown. c, Quantitative real-time PCR using primers for D1-R and D5-R to verify the differences in the expression of dopamine receptors on Teff and Treg. The results presented are arbitrary units and are from one of three representative experiments performed. d, e, Semiquantitative RT-PCR for D2-R, D3-R, and D4-R expression. mRNA was extracted from freshly purified Teff and Treg. The housekeeping gene β-actin was used for quantitative analysis. The results of one of five representative experiments are shown. f, Representative micrographs of D1-R-immunoreactive T-cells using fluorescence and confocal microscopy. Also shown are micrographs stained with Hoechst and visualized by fluorescence microscopy. D1-R immunoreactivity was observed in Treg but not in Teff.
Figure 4.
Molecular mechanism underlying the effect of dopamine on Treg. a, Expression of CTLA-4. Treg were activated for 24 hr, then incubated for 2 hr with dopamine or SKF-38393 (control cells were activated but were not incubated with either dopamine or SKF-38393; note that different cell preparations were used for each treatment, and therefore the controls used for each treatment were not the same) and were stained 24 hr later for CTLA-4 on cell surfaces. CTLA-4 expression was reduced after exposure to dopamine or to SKF-38393. Representative results of one of five independent experiments with each treatment are shown. b, Production of IL-10. Treg were activated for 24 hr with anti-CD3 and IL-2 in the presence of lethally irradiated splenocytes (APCs) and then for an additional 2 hr with dopamine. Conditioned media were collected after 24, 48, or 72 hr of culture and were assayed for IL-10 using a sandwich ELISA. At any given time, significantly less IL-10 was detected in media conditioned by dopamine-treated Treg than in media conditioned by Treg not exposed to dopamine. Statistical significance was verified using Student's t test analysis (**p < 0.01; *p < 0.05). The results shown are of one of three independent experiments, performed at each time point. c, Lack of IL-2 production by Treg. Treg and Teff were activated separately for 48 hr with anti-CD3 and anti-CD28 (without mrIL-2) with or without dopamine. Conditioned media were collected after 48 hr and subjected to ELISA. Treg with or without dopamine did not secrete detectable levels of IL-2. Production of IL-2 by Teff was not affected by dopamine. d, Foxp3 expression in Treg. Treg were activated for 24 hr with anti-CD3 and anti-CD28 in the presence of IL-2, then exposed to dopamine for 2 hr, washed, and analyzed 30 min later for Foxp3 expression. No changes in Foxp3 were detected after 30 min of dopamine treatment of naive Treg. DA, Dopamine.
Figure 5.
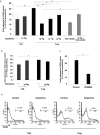
ERK1/2 phosphorylation inhibitors downregulate Treg-suppressive activity. a, Treg were activated by incubation for 30 min with anti-CD3 and anti-CD28 antibodies in the presence of IL-2 and in the presence or absence of a tyrosine kinase inhibitor (genistein) and were then cocultured with Teff. The suppression of Teff by Treg was significantly reduced in the presence of genistein. b, Similarly, incubation of activated Treg with the specific MEK inhibitor PD98059, which inhibits the ERK1/2 signaling pathway, almost completely abolished their suppression of Treg.
Figure 6.
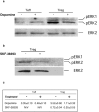
Correlation between Treg activity and activation state of ERK1/2. a,b, Western blot analyses of Treg lysates after activation for 20 min with anti-CD3 and anti-CD28, in the presence or absence of dopamine (a) or SKF-38393 (b). After activation, the amounts of phospho-ERK1/2 seen in Treg are larger than in Teff (a) but are reduced by dopamine (a) or by SKF-38393 (b). Dopamine did not cause a significant change in phospho-ERK1/2 levels in Teff (a, b). c, Quantitative analysis of phospho-bands using NIH Image version 1.62.
Figure 7.
Dopamine alters the adhesive properties of Treg. a, Treg and Teff were activated for 24 hr with anti-CD3 and anti-CD28 and were then incubated, with or without dopamine (10-5 to 10-9
m
), for 2 hr. In the absence of dopamine, adhesion of Treg to the CSPG matrix was significantly stronger than that of Teff. Incubation with dopamine significantly reduced the adhesion of Treg in a concentration-dependent manner. The effect of dopamine on Treg adhesion could be mimicked by SKF-38393, a specific agonist of the D1-type family. The dopamine effect was blocked by SCH-23390, a D1-type antagonist. Dopamine did not significantly alter the adhesion of Teff. A Mann-Whitney nonparametric U test was used for statistical analysis. b, In the absence of dopamine, adhesion of Treg to fibronectin was only slightly (but still significantly) stronger than that of Teff. However, dopamine did not significantly alter the adhesion of either Treg or Teff. A Mann-Whitney nonparametric U test was used for statistical analysis. c, Treg were activated for 30 min in the presence or absence of the ERK1/2 signaling pathway inhibitor PD98059 and then subjected to an adhesion assay to CSPG. Adhesion of Treg incubated with PD98059 was significantly weaker than that of control Treg cells. d, CD44 expression in Treg and in Teff. FACS analysis showed that significantly larger amounts of CD44 are expressed in Treg than in Teff. After incubation with dopamine, CD44 expression was significantly decreased in Treg but was not affected in Teff.
Figure 8.
Dopamine alters the migratory properties of Treg. a, The total population of purified CD4+ T-cells was subjected to a migration assay. The percentage of CD4+CD25+ T-cells in the total population after migration toward CCL22 (MDC) was significantly higher than in the original population. Exposure of Treg to dopamine significantly decreased their migration toward MDC. Migration of Teff toward SDF-1 was significantly stronger than that of Treg, and neither Teff nor Treg migration was affected by exposure to dopamine. A Mann-Whitney nonparametric U test was used for statistical analysis. b, c, Migration of purified Treg was significantly decreased after incubation of Treg with dopamine. Treg in the lower (postmigration) chamber were collected and counted by FACS for a defined time period after staining for a membrane CD4 marker. Values are representative results of the FACS analysis (b), and the mean number of cells from triplicates of the same experiment are shown in c. d, e, Semiquantitative reverse transcription-PCR for CCR-4 expression in Treg and Teff. mRNA was isolated from Teff and Treg, incubated for 2 hr with or without dopamine. The PCR products were quantified (e) relative to a housekeeping gene (β-actin). The results of one representative experiment are shown (d). Each experiment was performed in triplicate and repeated at least three times. DA, Dopamine. ***p < 0.01; **p < 0.01.
Figure 9.
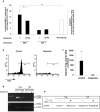
Systemic injection of dopamine, through downregulation Treg, activity increases neuronal survival after optic nerve crush injury. a, BALB/c mice were given injections of dopamine (0.4 mg/kg) immediately after being subjected to a partial crush injury of the optic nerve. Two weeks later, their retinas were excised and the numbers of surviving neurons were determined (see Materials and Methods). Significantly more neurons survived in dopamine-injected mice than in vehicle-injected controls (p < 0.01; Student's t test). Bars represent mean numbers of retinal ganglion cells per square meter of the retina. Each experiment was performed twice; n = 6-8 mice in each group. A two-tailed Student's t test was used for statistical analysis; ***p < 0.001; **p < 0.01. a, Neuronal survival was significantly worse in BALB/c mice that were inoculated (immediately after their exposure to a toxic excess of intraocular glutamate) with activated Treg than in Teff-inoculated mice. Neuronal loss is expressed as a percentage of the number of neurons in untreated glutamate-injected controls. Neuronal survival in BALB/c mice that were exposed to a toxic excess of intraocular glutamate and then treated with activated Treg that were incubated for 2 hr with 10-5
m
dopamine before being administered in vivo did not differ from that in vehicle-treated glutamate-injected mice. b, Representative micrographs of retinas from mice given injections of glutamate and either Teff or Treg. Each experiment was performed twice; n = 6-8 mice in each group. A two-tailed Student's t test was used for statistical analysis; ***p < 0.001.
Similar articles
- Lack of protective effect of local administration of triamcinolone or systemic treatment with methylprednisolone against damages caused by optic nerve crush in rats.
Huang TL, Chang CH, Lin KH, Sheu MM, Tsai RK. Huang TL, et al. Exp Eye Res. 2011 Feb;92(2):112-9. doi: 10.1016/j.exer.2010.12.008. Epub 2010 Dec 24. Exp Eye Res. 2011. PMID: 21185832 - Bacterial DNA confers neuroprotection after optic nerve injury by suppressing CD4+CD25+ regulatory T-cell activity.
Johnson TV, Camras CB, Kipnis J. Johnson TV, et al. Invest Ophthalmol Vis Sci. 2007 Aug;48(8):3441-9. doi: 10.1167/iovs.06-1351. Invest Ophthalmol Vis Sci. 2007. PMID: 17652711 - Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system.
Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Kipnis J, et al. Proc Natl Acad Sci U S A. 2002 Nov 26;99(24):15620-5. doi: 10.1073/pnas.232565399. Epub 2002 Nov 12. Proc Natl Acad Sci U S A. 2002. PMID: 12429857 Free PMC article. - Quantitative retinal protein analysis after optic nerve transection reveals a neuroprotective role for hepatoma-derived growth factor on injured retinal ganglion cells.
Hollander A, D'Onofrio PM, Magharious MM, Lysko MD, Koeberle PD. Hollander A, et al. Invest Ophthalmol Vis Sci. 2012 Jun 26;53(7):3973-89. doi: 10.1167/iovs.11-8350. Invest Ophthalmol Vis Sci. 2012. PMID: 22531700 - Thymic commitment of regulatory T cells is a pathway of TCR-dependent selection that isolates repertoires undergoing positive or negative selection.
Coutinho A, Caramalho I, Seixas E, Demengeot J. Coutinho A, et al. Curr Top Microbiol Immunol. 2005;293:43-71. doi: 10.1007/3-540-27702-1_3. Curr Top Microbiol Immunol. 2005. PMID: 15981475 Review.
Cited by
- Complement Has Brains-Do Intracellular Complement and Immunometabolism Cooperate in Tissue Homeostasis and Behavior?
Kunz N, Kemper C. Kunz N, et al. Front Immunol. 2021 Feb 25;12:629986. doi: 10.3389/fimmu.2021.629986. eCollection 2021. Front Immunol. 2021. PMID: 33717157 Free PMC article. Review. - Single-cell RNA-seq Reveals the Inhibitory Effect of Methamphetamine on Liver Immunity with the Involvement of Dopamine Receptor D1.
Zhou JT, Xu Y, Liu XH, Cheng C, Fan JN, Li X, Yu J, Li S. Zhou JT, et al. Genomics Proteomics Bioinformatics. 2024 Oct 15;22(4):qzae060. doi: 10.1093/gpbjnl/qzae060. Genomics Proteomics Bioinformatics. 2024. PMID: 39196711 Free PMC article. - A "Drug-Dependent" Immune System Can Compromise Protection against Infection: The Relationships between Psychostimulants and HIV.
Assis MA, Carranza PG, Ambrosio E. Assis MA, et al. Viruses. 2021 Apr 21;13(5):722. doi: 10.3390/v13050722. Viruses. 2021. PMID: 33919273 Free PMC article. Review. - Involvement of dopaminergic signaling in the cross talk between the renin-angiotensin system and inflammation.
Campos J, Pacheco R. Campos J, et al. Semin Immunopathol. 2020 Dec;42(6):681-696. doi: 10.1007/s00281-020-00819-8. Epub 2020 Sep 30. Semin Immunopathol. 2020. PMID: 32997225 Free PMC article. Review.
References
- Amin F, Davidson M, Kahn RS, Schmeidler J, Stern R, Knott PJ, Apter S (1995) Assessment of the central dopaminergic index of plasma HVA in schizophrenia. Schizophr Bull 21: 53-66. - PubMed
- Ariel A, Yavin EJ, Hershkoviz R, Avron A, Franitza S, Hardan I, Cahalon L, Fridkin M, Lider O (1998) IL-2 induces T cell adherence to extracellular matrix: inhibition of adherence and migration by IL-2 peptides generated by leukocyte elastase. J Immunol 161: 2465-2472. - PubMed
- Bretscher P, Cohn M (1970) A theory of self-nonself discrimination. Science 169: 1042-1049. - PubMed
- Chang WH, Chen TY, Lin SK, Lung FW, Lin WL, Hu WH, Yeh EK (1990) Plasma catecholamine metabolites in schizophrenics: evidence for the two-subtype concept. Biol Psychiatry 27: 510-518. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous