Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5alpha - PubMed (original) (raw)
Retrovirus resistance factors Ref1 and Lv1 are species-specific variants of TRIM5alpha
Theodora Hatziioannou et al. Proc Natl Acad Sci U S A. 2004.
Abstract
Mammalian cells express several factors that act in a cell-autonomous manner to inhibit retrovirus replication. Among these are the Friend virus susceptibility factor 1/lentivirus susceptibility factor 1/restriction factor 1 (Ref1) class of restriction factors, which block infection by targeting the capsids of diverse retroviruses. Here we show that lentivirus susceptibility factor 1 and Ref1 are species-specific variants of tripartite interaction motif 5alpha (TRIM5alpha), a cytoplasmic body component recently shown to block HIV-1 infection in rhesus macaque cells, and can indeed block infection by widely divergent retroviruses. Depletion of TRIM5alpha from human cells relieved restriction of N-tropic murine leukemia virus (N-MLV), and expression of human TRIM5alpha in otherwise nonrestricting cells conferred specific resistance to N-MLV infection, indicating that TRIM5alpha is Ref1 or an essential component of Ref1. TRIM5alpha variants from humans, rhesus monkeys, and African green monkeys displayed different but overlapping restriction specificities that were quite accurately predicted by the restriction properties of the cells from which they were derived. All TRIM5alpha variants could inhibit infection by at least two different retroviruses, and African green monkey TRIM5alpha was able to inhibit infection by no less than four divergent retroviruses of human, non-human primate, equine, and murine origin. However, each TRIM5alpha variant was unable to restrict retroviruses isolated from the same species. These data indicate that TRIM5alpha can confer broad innate immunity to retrovirus infection in primate cells and is likely to be an important natural barrier to cross-species retrovirus transmission.
Figures
Fig. 1.
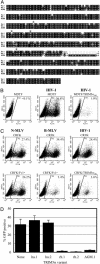
Rhesus monkey and AGM TRIM5α variants inhibit HIV-1 infection when expressed in murine or feline cells. (A) Alignment of human (Hu.1), rhesus (Rh.1), and AGM TRIM5α protein sequences. The asterisks indicate the positions of amino acids that differ in Hu.2 (R136Q) and Rh.2 (S184L, W196R, and T307P) alleles of TRIM5α.(B) Inhibition of HIV-1 infection by TRIM5αrh.1 in murine cells. MDTF cells that were either unmodified or stably expressing TRIM5αrh.1 were either mock-infected or infected with an HIV-1-GFP vector, as indicated. GFP expression measured in the FL1-H channel is plotted against side scatter (SSC-H). The percentage of cells falling within a gate (R2) that includes infected (GFP-positive) cells and excludes >99.9% of uninfected cells is indicated. (C) MLV-GFP or HIV-1-GFP infection of unmodified feline CRFK cells, or CRFK cells expressing Fv1n or TRIM5αrh.1, as indicated. (D) Effect of variant human, rhesus monkey, and AGM TRIM5α alleles, expressed in MDTF cells, on HIV-1 vector infection.
Fig. 2.
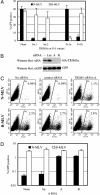
Human TRIM5α is necessary and sufficient to confer Ref1 activity. (A) Infection of MDTF cells that were unmodified (None) or expressing either of two human TRIM5α variants (hu.1 and hu.2) by N-MLV-GFP (filled bars) or B-MLV-GFP (open bars). The effects of Fv1n and Fv1b expression on N-MLV and B-MLV infection is shown for comparison. (B) Silencing of TRIM5αhu expression by using siRNA. HeLa cells were cotransfected with plasmids expressing HA-TRIM5α and GFP in the absence of siRNA (-) or in the presence of siRNAs targeting luciferase (Luc) or TRIM5αhu (lanes A and B). (C) Infection of human (HeLa) cells with N-MLV or B-MLV after transfection with either no siRNA, the control-luciferase-specific siRNA, or the TRIM5-specific siRNA-A, as indicated. (D) Effects of control (Luc) and TRIM5-specific siRNAs (A and B) on N-MLV (filled bars) and B-MLV (open bars) infection of HeLa cells.
Fig. 3.
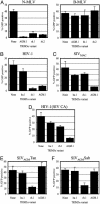
Non-human primate TRIM5α variants can inhibit infection by widely divergent retroviruses. (A) Infection of unmodified MDTF cells (None) or MDTF cells expressing AGM.1, rh.1, or rh.2 variants of TRIM5α by N-MLV (Left) and B-MLV (Right). (B_–_F) Infection of unmodified CRFK cells (None) or CRFK cells expressing hu.1, rh.1, or AGM.1 variants of TRIM5α, as indicated, by HIV-1 (B), SIVMAC (C), HIV-1(SIV CA) (D), SIVAGMTan (E), or SIVAGMSab (F).
Fig. 4.
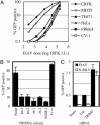
An equine retrovirus, EIAV, is inhibited by multiple human and non-human primate TRIM5α variants. (A) Titration of an EIAV-GFP vector on feline (CRFK), murine (MDTF), human (TE671 and HeLa), and non-human primate (FRhK4 and CV-1) cells. The percentage of GFP-positive cells as a function of inoculating EIAV–GFP dose, in CRFK infectious units (I.U.), is plotted. (B) Infection of unmodified CRFK cells (None) or CRFK cells expressing the indicated TRIM5α or Fv1 variants. (C) Infection of human (HeLa) cells with EIAV (filled bars) or N-MLV (open bars) after transfection with either no siRNA (None), the control-luciferase-specific siRNA (Luc), or the TRIM5-specific siRNA-A, as indicated.
Comment in
- In defense of the cell: TRIM5alpha interception of mammalian retroviruses.
Lee K, KewalRamani VN. Lee K, et al. Proc Natl Acad Sci U S A. 2004 Jul 20;101(29):10496-7. doi: 10.1073/pnas.0404066101. Epub 2004 Jul 13. Proc Natl Acad Sci U S A. 2004. PMID: 15252204 Free PMC article. No abstract available.
Similar articles
- The human and African green monkey TRIM5alpha genes encode Ref1 and Lv1 retroviral restriction factor activities.
Keckesova Z, Ylinen LM, Towers GJ. Keckesova Z, et al. Proc Natl Acad Sci U S A. 2004 Jul 20;101(29):10780-5. doi: 10.1073/pnas.0402474101. Epub 2004 Jul 12. Proc Natl Acad Sci U S A. 2004. PMID: 15249687 Free PMC article. - Isolation of an active Lv1 gene from cattle indicates that tripartite motif protein-mediated innate immunity to retroviral infection is widespread among mammals.
Ylinen LM, Keckesova Z, Webb BL, Gifford RJ, Smith TP, Towers GJ. Ylinen LM, et al. J Virol. 2006 Aug;80(15):7332-8. doi: 10.1128/JVI.00516-06. J Virol. 2006. PMID: 16840314 Free PMC article. - A novel envelope mediated post entry restriction of murine leukaemia virus in human cells is Ref1/TRIM5α independent.
Oliveira NM, Trikha R, McKnight Á. Oliveira NM, et al. Retrovirology. 2010 Oct 7;7:81. doi: 10.1186/1742-4690-7-81. Retrovirology. 2010. PMID: 20929586 Free PMC article. - Control of viral infectivity by tripartite motif proteins.
Towers GJ. Towers GJ. Hum Gene Ther. 2005 Oct;16(10):1125-32. doi: 10.1089/hum.2005.16.1125. Hum Gene Ther. 2005. PMID: 16218773 Free PMC article. Review. - Restriction factors: a defense against retroviral infection.
Bieniasz PD. Bieniasz PD. Trends Microbiol. 2003 Jun;11(6):286-91. doi: 10.1016/s0966-842x(03)00123-9. Trends Microbiol. 2003. PMID: 12823946 Review.
Cited by
- Crystal structure of TRIM20 C-terminal coiled-coil/B30.2 fragment: implications for the recognition of higher order oligomers.
Weinert C, Morger D, Djekic A, Grütter MG, Mittl PR. Weinert C, et al. Sci Rep. 2015 Jun 4;5:10819. doi: 10.1038/srep10819. Sci Rep. 2015. PMID: 26043233 Free PMC article. - HIV-1 Capsid Core: A Bullet to the Heart of the Target Cell.
Toccafondi E, Lener D, Negroni M. Toccafondi E, et al. Front Microbiol. 2021 Apr 1;12:652486. doi: 10.3389/fmicb.2021.652486. eCollection 2021. Front Microbiol. 2021. PMID: 33868211 Free PMC article. Review. - The human TRIM5alpha restriction factor mediates accelerated uncoating of the N-tropic murine leukemia virus capsid.
Perron MJ, Stremlau M, Lee M, Javanbakht H, Song B, Sodroski J. Perron MJ, et al. J Virol. 2007 Mar;81(5):2138-48. doi: 10.1128/JVI.02318-06. Epub 2006 Nov 29. J Virol. 2007. PMID: 17135314 Free PMC article. - TRIM5alpha selectively binds a restriction-sensitive retroviral capsid.
Sebastian S, Luban J. Sebastian S, et al. Retrovirology. 2005 Jun 20;2:40. doi: 10.1186/1742-4690-2-40. Retrovirology. 2005. PMID: 15967037 Free PMC article. - An active TRIM5 protein in rabbits indicates a common antiviral ancestor for mammalian TRIM5 proteins.
Schaller T, Hué S, Towers GJ. Schaller T, et al. J Virol. 2007 Nov;81(21):11713-21. doi: 10.1128/JVI.01468-07. Epub 2007 Aug 29. J Virol. 2007. PMID: 17728224 Free PMC article.
References
- Sheehy, A. M., Gaddis, N. C., Choi, J. D. & Malim, M. H. (2002) Nature 418, 646-650. - PubMed
- Harris, R. S., Bishop, K. N., Sheehy, A. M., Craig, H. M., Petersen-Mahrt, S. K., Watt, I. N., Neuberger, M. S. & Malim, M. H. (2003) Cell 113, 803-809. - PubMed
- Mangeat, B., Turelli, P., Caron, G., Friedli, M., Perrin, L. & Trono, D. (2003) Nature 424, 99-103. - PubMed
- Gao, G., Guo, X. & Goff, S. P. (2002) Science 297, 1703-1706. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous