p53 targets simian virus 40 large T antigen for acetylation by CBP - PubMed (original) (raw)
p53 targets simian virus 40 large T antigen for acetylation by CBP
Danielle L Poulin et al. J Virol. 2004 Aug.
Abstract
Simian virus 40 (SV40) large T antigen (T Ag) interacts with the tumor suppressor p53 and the transcriptional coactivators CBP and p300. Binding of these cellular proteins in a ternary complex has been implicated in T Ag-mediated transformation. It has been suggested that the ability of CBP/p300 to modulate p53 function underlies p53's regulation of cell proliferation and tumorigenesis. In this study, we provide further evidence that CBP activity may be mediated through its synergistic action with p53. We demonstrate that SV40 T Ag is acetylated in vivo in a p53-dependent manner and T Ag acetylation is largely mediated by CBP. The acetylation of T Ag is dependent on its interaction with p53 and on p53's interaction with CBP. We have mapped the site of acetylation on T Ag to the C-terminal lysine residue 697. This acetylation site is conserved between the T antigens of the human polyomaviruses JC and BK, which are also known to interact with p53. We show that both JC and BK T antigens are also acetylated at corresponding sites in vivo. While other proteins are known to be acetylated by CBP/p300, none are known to depend on p53 for acetylation. T Ag acetylation may provide a regulatory mechanism for T Ag binding to a cellular factor or play a role in another aspect of T Ag function.
Figures
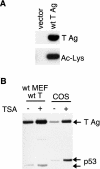
FIG. 1.
SV40 T Ag is acetylated in vivo. (A) Immunoprecipitations for T Ag from MEFs stably expressing T Ag or vector only were Western blotted with an antibody generated against acetylated lysine residues (bottom, Ac-Lys) or a T Ag antibody (top). (B) TSA treatment enhances T Ag acetylation. MEFs stably expressing T Ag or COS-1 cells were treated (+) or not treated (−) with 100 ng of TSA/ml for 2 h prior to lysis. T Ag was then immunoprecipitated with protein A-Sepharose beads cross-linked to a T Ag antibody and then Western blotted with an antibody generated against acetylated lysine residues.
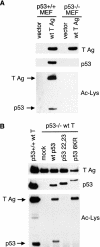
FIG. 2.
T Ag acetylation is dependent on p53. (A) T Ag acetylation is reduced in p53−/− MEFs. Whole-cell lysates from p53+/+ or p53−/− MEFs stably expressing T Ag or vector only were Western blotted for T Ag (top) and p53 (middle). T Ag was then immunoprecipitated from these cells and blotted with antibody generated against acetylated lysine residues (bottom). (B) Reintroduction of wt and 6KR mutant but not 22,23 mutant p53 restores T Ag acetylation in p53−/− MEFs. p53−/− MEFs were transfected with wt human p53, the CBP/p300 binding-deficient 22,23 mutant p53, and the acetylation site 6KR mutant p53. T Ag was immunoprecipitated from these cells and compared to T Ag immunoprecipitated from p53+/+ MEFs that stably express T Ag. Western blotting was performed for T Ag (top), p53 (middle), and Ac-Lys (bottom).
FIG. 3.
T Ag acetylation requires binding to p53 in both human and mouse cells. (A) T Ag is acetylated in p53+/+ but not p53−/− human cells. HCT116 cells were transfected with vector only or T Ag and Western blotted for T Ag (top) and p53 (middle). T Ag immunoprecipitations were blotted with an Ac-Lys antibody (bottom). (B) A p53-binding mutant T Ag is not acetylated. NIH 3T3 cells were transfected with wt T Ag or the p53-binding-deficient del434-444 mutant T Ag. T Ag was immunoprecipitated from these cells and compared to T Ag immunoprecipitated from wt MEFs that stably express wt T Ag. Western blotting was done for T Ag (top), p53 (middle), and Ac-Lys (bottom).
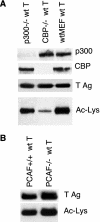
FIG. 4.
CBP specifically contributes to T Ag acetylation. (A) T Ag acetylation is reduced in CBP−/− but not p300−/− MEFs. T Ag was stably introduced into wt, p300−/−, and CBP−/− MEFs. Lysates were Western blotted for p300 (top) and CBP (second from top). T Ag was immunoprecipitated from these cells and blotted for T Ag (second from bottom) and Ac-Lys (bottom). (B) Levels of T Ag acetylation in PCAF+/+ and PCAF−/− MEFs are comparable. T Ag was immunoprecipitated from PCAF+/+ and PCAF−/− MEFs stably expressing T Ag and Western blotted for T Ag (top) or Ac-Lys (bottom).
FIG. 5.
The site of SV40 T Ag acetylation maps to the carboxy terminus. (A) Schematic representation of domains encompassed by various T Ag truncations. (B) The C terminus of T Ag (627 to 708) is required for T Ag acetylation. U2OS cells were transfected with various T Ag truncations as shown in panel A. HA immunoprecipitations were Western blotted (WB) with an HA antibody (top left) to show expression, p53 (bottom left), and Ac-Lys (right). (C) Lysine 697 is a potential site of T Ag acetylation. wt T and K697R, K698R, and K697,698R mutant T antigens were transfected in U2OS cells. T Ag was immunoprecipitated and then Western blotted for T Ag (top), p53 (middle), or Ac-Lys (bottom).
FIG. 6.
SV40 T Ag is acetylated on lysine residue 697. (A) An acetylation site-specific antibody generated against K697 was used to Western blot MEFs stably expressing wt T Ag or various acetylation site mutant T antigens, as well as NIH 3T3 cells and p53−/− MEFs that stably express wt T Ag. (B) The Ac-K697 antibody is effectively competed with acetylated K697 peptide in a dose-dependent manner but not with unacetylated K697 peptide. Lysates from MEFs stably expressing wt T Ag were Western blotted with the Ac-K697 antibody preincubated with either 0, 1, 2, or 5 μg of acetylated K697 peptide or 5 μg of unacetylated K697 peptide as indicated. The bottom blot shows equivalent levels of T Ag in all lanes.
FIG. 7.
The acetylation site of SV40 large T Ag is conserved with JC and BK large T antigens. (A) Alignment of C-terminal domains of SV40, BK, and JC large T antigens. Boxes, conserved residues; arrow, position of the conserved lysine residue corresponding to the acetylation site K697 on SV40 large T Ag. (B) BK and JC T antigens are acetylated on a conserved lysine residue. U2OS cells were transfected with SV40, BK, or JC T antigens and Western blotted for T Ag (top) and p53 (middle). Immunoprecipitations for T Ag were Western blotted with a site-specific acetylation antibody corresponding to the conserved lysine at position 697 on SV40 T Ag.
Similar articles
- Targeting of p300/CREB binding protein coactivators by simian virus 40 is mediated through p53.
Borger DR, DeCaprio JA. Borger DR, et al. J Virol. 2006 May;80(9):4292-303. doi: 10.1128/JVI.80.9.4292-4303.2006. J Virol. 2006. PMID: 16611888 Free PMC article. - Regulation of SV40 large T-antigen stability by reversible acetylation.
Shimazu T, Komatsu Y, Nakayama KI, Fukazawa H, Horinouchi S, Yoshida M. Shimazu T, et al. Oncogene. 2006 Nov 30;25(56):7391-400. doi: 10.1038/sj.onc.1209731. Epub 2006 Jun 12. Oncogene. 2006. PMID: 16767160 - Binding of p300/CBP co-activators by polyoma large T antigen.
Cho S, Tian Y, Benjamin TL. Cho S, et al. J Biol Chem. 2001 Sep 7;276(36):33533-9. doi: 10.1074/jbc.M102906200. Epub 2001 Jul 3. J Biol Chem. 2001. PMID: 11438528 - Cellular transformation by SV40 large T antigen: interaction with host proteins.
Ali SH, DeCaprio JA. Ali SH, et al. Semin Cancer Biol. 2001 Feb;11(1):15-23. doi: 10.1006/scbi.2000.0342. Semin Cancer Biol. 2001. PMID: 11243895 Review. - p300/CBP/p53 interaction and regulation of the p53 response.
Grossman SR. Grossman SR. Eur J Biochem. 2001 May;268(10):2773-8. doi: 10.1046/j.1432-1327.2001.02226.x. Eur J Biochem. 2001. PMID: 11358491 Review.
Cited by
- Histone deacetylase III interactions with BK polyomavirus large tumor antigen may affect protein stability.
Hsu YH, Chao CN, Huang HY, Zhao PW, Hsu PH, Shen CH, Chen SY, Fang CY. Hsu YH, et al. Virol J. 2023 Jul 18;20(1):155. doi: 10.1186/s12985-023-02128-6. Virol J. 2023. PMID: 37464367 Free PMC article. - Contribution of DNA Replication to the FAM111A-Mediated Simian Virus 40 Host Range Phenotype.
Tarnita RM, Wilkie AR, DeCaprio JA. Tarnita RM, et al. J Virol. 2018 Dec 10;93(1):e01330-18. doi: 10.1128/JVI.01330-18. Print 2019 Jan 1. J Virol. 2018. PMID: 30333173 Free PMC article. - Human BK Polyomavirus-The Potential for Head and Neck Malignancy and Disease.
Burger-Calderon R, Webster-Cyriaque J. Burger-Calderon R, et al. Cancers (Basel). 2015 Jul 8;7(3):1244-70. doi: 10.3390/cancers7030835. Cancers (Basel). 2015. PMID: 26184314 Free PMC article. Review. - Phosphorylation of Merkel cell polyomavirus large tumor antigen at serine 816 by ATM kinase induces apoptosis in host cells.
Li J, Diaz J, Wang X, Tsang SH, You J. Li J, et al. J Biol Chem. 2015 Jan 16;290(3):1874-84. doi: 10.1074/jbc.M114.594895. Epub 2014 Dec 5. J Biol Chem. 2015. PMID: 25480786 Free PMC article. - Removal of a small C-terminal region of JCV and SV40 large T antigens has differential effects on transformation.
Seneca NTM, Sáenz Robles MT, Pipas JM. Seneca NTM, et al. Virology. 2014 Nov;468-470:47-56. doi: 10.1016/j.virol.2014.07.038. Epub 2014 Aug 16. Virology. 2014. PMID: 25129438 Free PMC article.
References
- Ait-Si-Ali, S., S. Ramirez, F. X. Barre, F. Dkhissi, L. Magnaghi-Jaulin, J. A. Girault, P. Robin, M. Knibiehler, L. L. Pritchard, B. Ducommun, D. Trouche, and A. Harel-Bellan. 1998. Histone acetyltransferase activity of CBP is controlled by cycle-dependent kinases and oncoprotein E1A. Nature 396:184-186. - PubMed
- Ali, S. H., and J. A. DeCaprio. 2001. Cellular transformation by SV40 large T antigen: interaction with host proteins. Semin. Cancer Biol. 11:15-23. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous