Expression of histone deacetylase 8, a class I histone deacetylase, is restricted to cells showing smooth muscle differentiation in normal human tissues - PubMed (original) (raw)
Expression of histone deacetylase 8, a class I histone deacetylase, is restricted to cells showing smooth muscle differentiation in normal human tissues
David Waltregny et al. Am J Pathol. 2004 Aug.
Abstract
Histone deacetylases (HDACs) were originally identified as nuclear enzymes involved in gene transcription regulation. Until recently, it was thought that their activity was restricted within the nucleus, with histones as unique substrates. The demonstration that specific HDACs deacetylate nonhistone proteins, such as p53 and alpha-tubulin, broadened the field of activity of these enzymes. HDAC8, a class I HDAC, is considered to be ubiquitously expressed, as suggested by results of Northern blots performed on tissue RNA extracts, and transfection experiments using various cell lines have indicated that this enzyme may display a prominent nuclear localization. Using immunohistochemistry, we unexpectedly found that, in normal human tissues, HDAC8 is exclusively expressed by cells showing smooth muscle differentiation, including visceral and vascular smooth muscle cells, myoepithelial cells, and myofibroblasts, and is mainly detected in their cytosol. These findings were confirmed in vitro by nucleo-cytoplasmic fractionation and immunoblot experiments performed on human primary smooth muscle cells, and by the cytosolic detection of epitope-tagged HDAC8 overexpressed in fibroblasts. Immunocytochemistry strongly suggested a cytoskeleton-like distribution of the enzyme. Further double-immunofluorescence staining experiments coupled with confocal microscopy analysis showed that epitope-tagged HDAC8 overexpressed in murine fibroblasts formed cytoplasmic stress fiber-like structures that co-localized with the smooth muscle cytoskeleton protein smooth muscle alpha-actin. Our works represent the first demonstration of the restricted expression of a class I HDAC to a specific cell type and indicate that HDAC8, besides being a novel marker of smooth muscle differentiation, may play a role in the biology of these contractile cells.
Figures
Figure 1
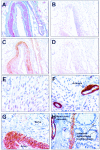
A: Masson’s trichrome staining of arterial walls present in the fat tissue surrounding a human prostate gland. Smooth muscle fibers appeared violet and collagen fibers were stained in green. HDAC8 expression was analyzed in serial tissue sections of the same arterial walls as in A using an immunoperoxidase technique, as described in Materials and Methods. B: Control immunohistochemical experiment in which the anti-HDAC8 antibody had been replaced with PBS. C: Incubation of the section with anti-HDAC8 antibody yielded a specific intense immunoreactivity in smooth muscle cells from the arterial walls. D: Control experiment in which anti-HDAC8 antibody had been preincubated with a molar excess of the corresponding peptide. E: Immunohistochemical detection of HDAC5 in the nucleus of human cardiomyocytes. F: Detection of HDAC8 by immunohistochemistry in myocardium. Neither cardiomyocytes nor endothelial cells from small arteries exhibited a detectable level of HDAC8 expression whereas vascular smooth muscle cells harbored strong anti-HDAC8 immunoreactivity. Note a detectable level of HDAC8 expression in a small capillary wall. G: Strong diffuse cytoplasmic anti-HDAC8 immunoreactivity in smooth muscle cells from a small artery wall in a liver portal space. Note the absence of specific reactivity in an adjacent nerve. H: Strong diffuse anti-HDAC8 reactivity in the cytosol of smooth muscle cells from colon muscularis mucosae and from arterial and capillary walls. Note detectable HDAC8 expression in scattered cells with myofibroblastic features (intestinal subepithelial myofibroblasts) located in the lamina propria. Tissue sections were counterstained with hematoxylin. Original magnifications: ×100 (A–D); ×200 (E, F); ×400 (G, H).
Figure 2
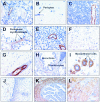
Immunodetection of HDAC8 in various human organs and tissues. Formalin-fixed, paraffin-embedded human tissue sections were subjected to detection of HDAC8 by immunoperoxidase, as described in Materials and Methods. HDAC8 expression was investigated in thyroid (A), brain (B), thymus (C), testis (D), pancreas (E), liver (hepatocytes) (F), skeletal muscle (G), lung (H), breast (I), uterine cervix (J), fallopian tube (K), and bladder wall (L). Sections were counterstained with hematoxylin. Original magnifications: ×100 (C, H); ×200 (J, K, L); ×400 (A, B, E, G); ×630 (D, F, I).
Figure 3
Immunodetection of HDAC8, α-SMA, and SMMHC in myoepithelial cells from normal human tracheal mucous glands and mammary acini/ducts. Serial sections of these tissues were subjected to detection of HDAC8, α-SMA, and SMMHC by immunoperoxidase, as described in Materials and Methods. Original magnifications, ×400.
Figure 4
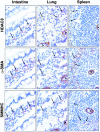
Immunodetection of HDAC8, α-SMA, and SMMHC in myofibroblastic cells from normal human intestine, lung, and spleen. Intestinal subepithelial myofibroblasts, lung alveolar septae myofibroblasts, and spleen reticular cells co-expressed HDAC8, α-SMA, and SMMHC. Serial sections of these tissues were subjected to detection of HDAC8, α-SMA, and SMMHC by immunoperoxidase, as described in Materials and Methods. v = vessel; arrows indicate myofibroblastic cells. Original magnifications, ×400.
Figure 5
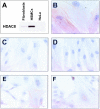
A: Protein lysates from primary human skin fibroblasts, primary HSMCs, and HeLa human cervix epithelial cells were subjected to immunoblot analysis of HDAC8 expression, as described in Materials and Methods. HDAC8 expression was investigated in human primary skin fibroblasts and smooth muscle cells from human umbilical cord vein (HSMC), by immunoperoxidase, as described in Materials and Methods. B: Anti-HDAC8 immunostaining presented a pattern suggestive of a cytoskeletal association in HSMCs. C: Anti-HDAC8 immunoreactivity in HSMCs was abolished by preincubating anti-HDAC8 antibody with a molar excess of the corresponding peptide. D: Primary human skin fibroblasts usually exhibited no detectable level of anti-HDAC8 immunoreactivity. E and F: Detection of HDAC8 in the cytoplasm (E) or nucleus (F) of NIH-3T3 cells. Original magnifications: ×630 (B); ×400 (C, D); ×200 (E, F).
Figure 6
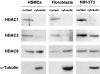
The amount of HDAC1, HDAC3, HDAC8, and α-tubulin in the fractionated nuclear and cytosolic extracts from primary human skin fibroblasts, HSMCs, and NIH-3T3 fibroblasts was analyzed by immunoblotting as described in Materials and Methods.
Figure 7
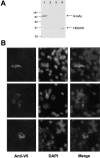
A: Western blot analysis of transfected recombinant human HDAC8 in NIH-3T3 cells. Total cell extracts from NIH-3T3 cells transiently transfected with pcDNA3.1D/HDAC8/V5-His, as in Materials and Methods, were subjected to immunoblot analysis using an anti-V5 antibody (Invitrogen). Lane designations were as follows: lane 1, NIH-3T3 transiently transfected with pcDNA3.1D/lacZ/V5-His; lane 2, NIH-3T3 transiently transfected with the insert-less vector; lane 3, untransfected NIH-3T3 cells; lane 4, NIH-3T3 transiently transfected with pcDNA3.1D/HDAC8/V5-His. B: Subcellular localization of V5-tagged HDAC8 transfected into NIH-3T3 cells. Left: Representative examples of anti-V5 immunofluorescence, as described in Materials and Methods. Middle and right: 4′,6′-diamidino-2′-phenylindole dichloride and merged images, respectively.
Figure 8
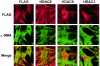
Double-immunofluorescence staining coupled with confocal microscopy analysis, using anti-α-SMA and anti-FLAG antibodies after transfection of cDNAs encoding either HDAC3, HDAC6, or HDAC8 (C-terminal FLAG-tagged) in NIH-3T3 cells. NIH-3T3 cells grown on coverslips were transfected with pcDNA3.1(+)/HDAC3/FLAG, pcDNA3.1(+)/HDAC6/FLAG, or pcDNA3.1(+)/HDAC8/FLAG. NIH-3T3 cells transfected with pcDNA3.1(+)/FLAG alone served as negative control. Transfected cells were processed for immunofluorescence microscopy 48 hours after transfection and analyzed using confocal microscopy as described in Materials and Method_s_.
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.
Ryan R, Hill S. Ryan R, et al. Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article. - Antioxidants for female subfertility.
Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Showell MG, et al. Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD007807. doi: 10.1002/14651858.CD007807.pub4. Cochrane Database Syst Rev. 2020. PMID: 32851663 Free PMC article. - Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.
Love AMA, Edwards C, Cai RY, Gibbs V. Love AMA, et al. Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article. - Antioxidants for female subfertility.
Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Showell MG, et al. Cochrane Database Syst Rev. 2017 Jul 28;7(7):CD007807. doi: 10.1002/14651858.CD007807.pub3. Cochrane Database Syst Rev. 2017. PMID: 28752910 Free PMC article. Updated. Review.
Cited by
- Targeting Histone Deacetylases in Idiopathic Pulmonary Fibrosis: A Future Therapeutic Option.
Korfei M, Mahavadi P, Guenther A. Korfei M, et al. Cells. 2022 May 12;11(10):1626. doi: 10.3390/cells11101626. Cells. 2022. PMID: 35626663 Free PMC article. Review. - Evaluation of 6-([(18)F]fluoroacetamido)-1-hexanoicanilide for PET imaging of histone deacetylase in the baboon brain.
Reid AE, Hooker J, Shumay E, Logan J, Shea C, Kim SW, Collins S, Xu Y, Volkow N, Fowler JS. Reid AE, et al. Nucl Med Biol. 2009 Apr;36(3):247-58. doi: 10.1016/j.nucmedbio.2008.12.005. Nucl Med Biol. 2009. PMID: 19324270 Free PMC article. - Analysis of the interactome of Schistosoma mansoni histone deacetylase 8.
Caby S, Pagliazzo L, Lancelot J, Saliou JM, Bertheaume N, Pierce RJ, Roger E. Caby S, et al. PLoS Negl Trop Dis. 2017 Nov 20;11(11):e0006089. doi: 10.1371/journal.pntd.0006089. eCollection 2017 Nov. PLoS Negl Trop Dis. 2017. PMID: 29155817 Free PMC article. - Expression of the class 1 histone deacetylases HDAC8 and 3 are associated with improved survival of patients with metastatic melanoma.
Wilmott JS, Colebatch AJ, Kakavand H, Shang P, Carlino MS, Thompson JF, Long GV, Scolyer RA, Hersey P. Wilmott JS, et al. Mod Pathol. 2015 Jul;28(7):884-94. doi: 10.1038/modpathol.2015.34. Epub 2015 Apr 3. Mod Pathol. 2015. PMID: 25836739 - Origin and differentiation of vascular smooth muscle cells.
Wang G, Jacquet L, Karamariti E, Xu Q. Wang G, et al. J Physiol. 2015 Jul 15;593(14):3013-30. doi: 10.1113/JP270033. Epub 2015 Jun 9. J Physiol. 2015. PMID: 25952975 Free PMC article. Review.
References
- Megee PC, Morgan BA, Mittman BA, Smith MM. Genetic analysis of histone H4: essential role of lysines subject to reversible acetylation. Science. 1990;247:841–845. - PubMed
- Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. - PubMed
- Nagy L, Kao HY, Chakravarti D, Lin RJ, Hassig CA, Ayer DE, Schreiber SL, Evans RM. Nuclear receptor repression mediated by a complex containing SMRT, mSin3A, and histone deacetylase. Cell. 1997;89:373–380. - PubMed
- Alland L, Muhle R, Hou H, Jr, Potes J, Chin L, Schreiber-Agus N, DePinho RA. Role for N-CoR and histone deacetylase in Sin3-mediated transcriptional repression. Nature. 1997;387:49–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous