Differential effect of bicycling exercise intensity on activity and phosphorylation of atypical protein kinase C and extracellular signal-regulated protein kinase in skeletal muscle - PubMed (original) (raw)
Comparative Study
Differential effect of bicycling exercise intensity on activity and phosphorylation of atypical protein kinase C and extracellular signal-regulated protein kinase in skeletal muscle
Erik A Richter et al. J Physiol. 2004.
Abstract
Atypical protein kinase C (aPKC) and extracellular signal-regulated kinase (ERK) are emerging as important signalling molecules in the regulation of metabolism and gene expression in skeletal muscle. Exercise is known to increase activity of aPKC and ERK in skeletal muscle but the effect of exercise intensity hereon has not been studied. Furthermore, the relationship between activity and phosphorylation of the two enzymes during exercise is unknown. Nine healthy young men exercised for 30 min on a bicycle ergometer on two occasions. One occasion consisted of three consecutive 10 min bouts of 35, 60 and 85% of peak pulmonary oxygen uptake V(O(2 peak)) and the second of one 30 min bout at 35% of V(O(2 peak)). Both trials also included 30 min recovery. Muscle biopsies were obtained from the vastus lateralis muscle before and after each exercise bout. Exercise increased muscle aPKC activity at 35% V(O(2 peak)), whereupon no further increase was observed at higher exercise intensities. Activation of aPKC was not accompanied by increased phosphorylation of aPKC Thr(410/403). ERK1/2 activity increased in a similar pattern to aPKC, reaching maximal activity at 35% V(O(2 peak)), whereas ERK1 Thr(202)/Tyr(204) and ERK2 Thr(183)/Tyr(185) phosphorylation increased with increasing exercise intensity. Thus, aPKC and ERK1/2 activity in muscle during exercise did not correspond to phosphorylation of sites on aPKC or ERK1/2, respectively, which are considered important for their activation. It is concluded that assessment of aPKC and ERK1/2 activity in muscle using phosphospecific antibodies did not reflect direct activity measurements on immunoprecipitated enzyme in vitro. Thus, estimation of enzyme activity during exercise by use of phosphospecific antibodies should not be performed uncritically. In addition, increase in muscle activity of aPKC or ERK1/2 during exercise is not closely related to energy demands of the muscle but may serve other regulatory or permissive functions in muscle.
Figures
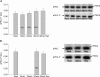
Figure 1. Phosphospecificity of aPKC-antibody
A shows blots of lysates prepared from human vastus lateralis muscle probed with the antibody against atypical PKC (aPKC) phosphorylated on Thr410/403. Same sample was loaded 4 times in 2 different amounts. B shows complete absence of signal after treatment for 2 h of the membrane with 500 U ml−1 λ-protein phosphatase before probing with the aPKC Thr410/403 phosphospecific antibody. C and D show blots for aPKC in its nonphosphorylated form without (C) and with (D) phosphatase treatment. This signal was unaffected by phosphatase treatment.
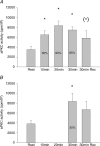
Figure 2. aPKC activity
A, activity of aPKC in vastus lateralis muscle at rest, immediately after each 10 min sequential exercise bout of 35, 60 and 85% of _V̇_O2peak and after 30 min recovery. Values are means ±
s.e.m.
of 9 determinations. B, aPKC activity in vastus lateralis muscle at rest, after 30 min exercise at 35% of _V̇_O2peak and after 30 min recovery. Values are means ±
s.e.m.
of 9 determinations. *P < 0.05 compared to rest; (*)P < 0.1 compared to rest.
Figure 3. aPKC phosphorylation
A, phosphorylation of aPKC Thr410/403 in vastus lateralis muscle at rest, immediately after each 10 min sequential exercise bout at 35, 60 and 85% of _V̇_O2peak and after 30 min recovery. Representative Western blots of both aPKC Thr410/403 phosphorylation and aPKC protein are shown in the inset. Values are means ±
s.e.m.
of 9 determinations and are expressed in arbitrary units relative to aPKC protein content in each sample. B, phosphorylation of aPKC Thr410/403 in vastus lateralis muscle at rest, after 30 min exercise at 35% of _V̇_O2peak and after 30 min recovery. Representative Western blots of both aPKC Thr410/403 phosphorylation and aPKC protein are also shown. Values are means ±
s.e.m.
of 9 determinations and are expressed in arbitrary units relative to aPKC protein content in each sample.
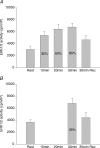
Figure 4. ERK1/2 activity
A, activity of ERK1/2 in vastus lateralis muscle at rest, immediately after each 10 min sequential exercise bout of 35, 60 and 85% of _V̇_O2peak and after 30 min recovery. Values are means ±
s.e.m.
of 9 determinations. B, activity of ERK1/2 in vastus lateralis muscle at rest, after 30 min exercise at 35% of _V̇_O2peak and after 30 min recovery. Values are means ±
s.e.m.
of 9 determinations. *P < 0.05 compared to rest.
Figure 5. ERK1/2 phosphorylation
A, phosphorylation of ERK1/2 in vastus lateralis muscle at rest, immediately after each 10 min sequential exercise bout of 35, 60 and 85% of _V̇_O2peak and after 30 min recovery. Representative Western blots of both ERK1 Thr202/Tyr204 and ERK2 Thr183/Tyr185 and ERK1/2 protein are also shown. Values are means ±
s.e.m.
of 9 determinations and are expressed in arbitrary units relative to ERK1/2 protein content in each sample. B, phosphorylation of ERK1/2 in vastus lateralis muscle at rest, after 30 min exercise at 35% **V̇**O2peak and after 30 min recovery. Representative Western blots of both phosphorylation of ERK1 Thr202/Tyr204, ERK2 Thr183/Tyr185 and ERK1/2 protein are also shown. Values are means ±
s.e.m.
of 9 determinations and are expressed in arbitrary units relative to ERK1/2 protein content in each sample. *P < 0.05 compared to rest; (*)P < 0.1 compared to rest; ‡P < 0.05 compared to other trial; #P < 0.05 compared to rest and recovery; and §P < 0.05 compared to rest, exercise at 35 and 60% of _V̇_O2peak and recovery.
Similar articles
- Effect of exercise on protein kinase C activity and localization in human skeletal muscle.
Rose AJ, Michell BJ, Kemp BE, Hargreaves M. Rose AJ, et al. J Physiol. 2004 Dec 15;561(Pt 3):861-70. doi: 10.1113/jphysiol.2004.075549. Epub 2004 Oct 7. J Physiol. 2004. PMID: 15604232 Free PMC article. - AICAR and metformin, but not exercise, increase muscle glucose transport through AMPK-, ERK-, and PDK1-dependent activation of atypical PKC.
Sajan MP, Bandyopadhyay G, Miura A, Standaert ML, Nimal S, Longnus SL, Van Obberghen E, Hainault I, Foufelle F, Kahn R, Braun U, Leitges M, Farese RV. Sajan MP, et al. Am J Physiol Endocrinol Metab. 2010 Feb;298(2):E179-92. doi: 10.1152/ajpendo.00392.2009. Epub 2009 Nov 3. Am J Physiol Endocrinol Metab. 2010. PMID: 19887597 Free PMC article. - Metabolic and mitogenic signal transduction in human skeletal muscle after intense cycling exercise.
Yu M, Stepto NK, Chibalin AV, Fryer LG, Carling D, Krook A, Hawley JA, Zierath JR. Yu M, et al. J Physiol. 2003 Jan 15;546(Pt 2):327-35. doi: 10.1113/jphysiol.2002.034223. J Physiol. 2003. PMID: 12527721 Free PMC article. - Exercise improves phosphatidylinositol-3,4,5-trisphosphate responsiveness of atypical protein kinase C and interacts with insulin signalling to peptide elongation in human skeletal muscle.
Frøsig C, Sajan MP, Maarbjerg SJ, Brandt N, Roepstorff C, Wojtaszewski JF, Kiens B, Farese RV, Richter EA. Frøsig C, et al. J Physiol. 2007 Aug 1;582(Pt 3):1289-301. doi: 10.1113/jphysiol.2007.136614. Epub 2007 May 31. J Physiol. 2007. PMID: 17540697 Free PMC article. - Effects of resistance exercise intensity on extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase activation in men.
Taylor LW, Wilborn CD, Kreider RB, Willoughby DS. Taylor LW, et al. J Strength Cond Res. 2012 Mar;26(3):599-607. doi: 10.1519/JSC.0b013e318242f92d. J Strength Cond Res. 2012. PMID: 22343976 Clinical Trial.
Cited by
- Contraction stimulates muscle glucose uptake independent of atypical PKC.
Yu H, Fujii NL, Toyoda T, An D, Farese RV, Leitges M, Hirshman MF, Mul JD, Goodyear LJ. Yu H, et al. Physiol Rep. 2015 Nov;3(11):e12565. doi: 10.14814/phy2.12565. Physiol Rep. 2015. PMID: 26564060 Free PMC article. - Skeletal muscle signaling response to sprint exercise in men and women.
Fuentes T, Guerra B, Ponce-González JG, Morales-Alamo D, Guadalupe-Grau A, Olmedillas H, Rodríguez-García L, Feijoo D, De Pablos-Velasco P, Fernández-Pérez L, Santana A, Calbet JA. Fuentes T, et al. Eur J Appl Physiol. 2012 May;112(5):1917-27. doi: 10.1007/s00421-011-2164-0. Epub 2011 Sep 18. Eur J Appl Physiol. 2012. PMID: 21928060 - Post-translational regulation of muscle growth, muscle aging and sarcopenia.
Zhong Q, Zheng K, Li W, An K, Liu Y, Xiao X, Hai S, Dong B, Li S, An Z, Dai L. Zhong Q, et al. J Cachexia Sarcopenia Muscle. 2023 Jun;14(3):1212-1227. doi: 10.1002/jcsm.13241. Epub 2023 May 1. J Cachexia Sarcopenia Muscle. 2023. PMID: 37127279 Free PMC article. Review. - Effects of exercise on AMPK signaling and downstream components to PI3K in rat with type 2 diabetes.
Cao S, Li B, Yi X, Chang B, Zhu B, Lian Z, Zhang Z, Zhao G, Liu H, Zhang H. Cao S, et al. PLoS One. 2012;7(12):e51709. doi: 10.1371/journal.pone.0051709. Epub 2012 Dec 13. PLoS One. 2012. PMID: 23272147 Free PMC article. - Effect of exercise on protein kinase C activity and localization in human skeletal muscle.
Rose AJ, Michell BJ, Kemp BE, Hargreaves M. Rose AJ, et al. J Physiol. 2004 Dec 15;561(Pt 3):861-70. doi: 10.1113/jphysiol.2004.075549. Epub 2004 Oct 7. J Physiol. 2004. PMID: 15604232 Free PMC article.
References
- Aronson D, Wojtaszewski JF, Thorell A, Nygren J, Zangen D, Richter EA, Ljungqvist O, Fielding RA, Goodyear LJ. Extracellular-regulated protein kinase cascades are activated in response to injury in human skeletal muscle. Am J Physiol. 1998;275:C555–C561. - PubMed
- Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert ML, Quon MJ, Reed BC, Dikic I, Farese RV. Glucose activates protein kinase C zeta/lambda through proline-rich tyrosine kinase-2, extracellular signal-regulated kinase, and phospholipase D: a novel mechanism for activating glucose transporter translocation. J Biol Chem. 2001;276:35537–35545. - PubMed
- Bandyopadhyay G, Standaert ML, Sajan MP, Karnitz LM, Cong L, Quon MJ, Farese RV. Dependence of insulin-stimulated glucose transporter 4 translocation on 3-phosphoinositide-dependent protein kinase-1 and its target threonine-410 in the activation loop of protein kinase C-{zeta} Mol Endocrinol. 1999;13:1766–1772. - PubMed
- Beeson M, Sajan MP, Dizon M, Grebenev D, Gomez-Daspet J, Miura A, Kanoh Y, Powe J, Bandyopadhyay G, Standaert ML, Farese RV. Activation of protein kinase C-zeta by insulin and phosphatidylinositol-3,4,5-(PO4) 3 is defective in muscle in type 2 diabetes and impaired glucose tolerance: amelioration by rosiglitazone and exercise. Diabetes. 2003;52:1926–1934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous