Interleukin-18 promotes joint inflammation and induces interleukin-1-driven cartilage destruction - PubMed (original) (raw)
Comparative Study
Interleukin-18 promotes joint inflammation and induces interleukin-1-driven cartilage destruction
Leo A B Joosten et al. Am J Pathol. 2004 Sep.
Abstract
Interleukin (IL)-18 is a member of the IL-1 family of proteins that exerts proinflammatory effects and is a pivotal cytokine for the development of Th1 responses. The goal of the present study was to investigate whether IL-18 induces joint inflammation and joint destruction directly or via induction of other cytokines such as IL-1 and tumor necrosis factor (TNF). To this end we performed both in vitro and in vivo kinetic studies. For in vivo IL-18 exposure studies C57BL/6, TNF-deficient, and IL-1-deficient mice were injected intra-articularly with 1.10(7) pfu mIL-18 adenovirus followed by histopathological examination. Local overexpression of IL-18 resulted in pronounced joint inflammation and cartilage proteoglycan loss in control mice. Of high interest, IL-18 gene transfer in IL-1-deficient mice did not show cartilage damage, although joint inflammation was similar to that in wild-type animals. Overexpression of IL-18 in TNF-deficient mice showed that TNF was partly involved in IL-18-induced joint swelling and influx of inflammatory cells, but cartilage proteoglycan loss occurred independent of TNF. In vitro cartilage degradation by IL-18 was found after a 72-hour culture period. Blocking of IL-1 with IL-1Ra or an ICE-inhibitor resulted in complete protection against IL-18-mediated cartilage degradation. The present study demonstrated that IL-18 induces joint inflammation independently of IL-1. In addition, we showed that IL-1beta generation, because of IL-18 exposure, was essential for marked cartilage degradation both in vitro and in vivo. These findings implicate that IL-18, in contrast to TNF, contributes through separate pathways to joint inflammation and cartilage destruction.
Figures
Figure 1
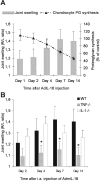
IL-18-driven joint inflammation in wild-type, IL-1−/−, and TNF−/− mice. IL-18 was overexpressed in the right knee of C57/BL6 mice by intra-articular injection of 1.107 pfu of AdmIL-18. A: At several time points both joint swelling (99mTc-uptake) and chondrocyte proteoglycan synthesis (35S-sulfate incorporation) were assessed. B: To examine the role of either IL-1 or TNF, IL-18 was overexpressed in IL-1- or TNF-deficient mice. Data expressed the mean ± SD of at least eight mice per group. The experiment was repeated once with approximately the same outcome. *, P < 0.01, Mann-Whitney _U_-test compared to C57/BL6 wild-type mice control group.
Figure 2
Joint inflammation induced by prolonged IL-18 overexpression. At day 0 1.107 pfu of control virus (Ad5del70-3) or 1.107 pfu of AdmIL-18 was intra-articularly injected in wild-type, IL-1-deficient, or TNF-deficient mice. At day 7 knee joints were removed and processed for histological analysis. A: Inflammation found after injection of Ad5del70-3 control virus. B: IL-18-induced joint inflammation in a wild-type mouse. C: IL-18-induced joint inflammation in IL-1 gene-deficient mouse. D: IL-18-induced joint inflammation in TNF knockout mouse. P, patella; F, femur; JC, joint cavity; and C, cartilage. H&E staining. Original magnifications, ×200.
Figure 3
Cartilage degradation after IL-18 gene transfer in wild-type, IL-1-deficient, or TNF-deficient mice. IL-18-driven cartilage proteoglycan loss was analyzed on day 14 after intra-articular injection of 1.107 pfu of Ad5del70-3 or AdmIL-18. A: Wild-type mouse injected with control virus. B: AdmIL-18 injection in a wild-type mouse. C: IL-18 overexpression in a IL-1α,β-deficient mouse. D: IL-18 overexpression in a TNF-deficient mouse. For details see Figure 2. Note the enhanced cartilage proteoglycan loss in wild-type and TNF-deficient mice (arrows). Safranin O staining. Original magnifications, ×400.
Figure 4
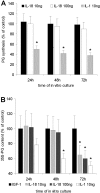
Prolonged exposure to IL-18 induced cartilage degradation in vitro. A: Patellar cartilage explants were isolated from naïve knee joints of C57/BL6 mice and cultured for 24 to 72 hours in RPMI 1640 medium supplemented with recombinant hIGF-1 (250 ng/ml) with or without mIL-18. Thereafter chondrocyte proteoglycan synthesis was determined by 35S-sulfate incorporation. For details see Materials and Methods. B: For cartilage degradation studies cartilage explants were prelabeled with 35S-sulfate. Data expressed the mean ± SD of six patellae per group. The experiments were repeated twice with similar outcome. *, P < 0.01, Mann-Whitney _U_-test compared to IGF-1 control group.
Figure 5
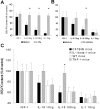
In vitro cartilage degradation by IL-18 is mediated by IL-1. Patellar cartilage explants were prelabeled as indicated in Materials and Methods. Thereafter, cartilage explants were cultured for 72 hours with IGF-1-containing medium with either IL-18 (10 or 100 ng/ml) or IL-1β (10 ng/ml). To block IL-1 we added either recombinant mIL-1Ra (A, 10 μg/ml) or ICE-inhibitor (B, 2.5 μmol/L) to the culture medium. C: To confirm the ICE data and examine the role of TNF in IL-18-driven proteoglycan loss we use patellar explants from both IL-1β- and TNF-α-deficient mice. For details see Figure 4. *, P < 0.01, Mann-Whitney _U_-test compared to C57/BL6 × 129Sv wild-type mice control group.
Similar articles
- Major role for interleukin 1 but not for tumor necrosis factor in early cartilage damage in immune complex arthritis in mice.
Van Lent PL, Van De Loo FA, Holthuysen AE, Van Den Bersselaar LA, Vermeer H, Van Den Berg WB. Van Lent PL, et al. J Rheumatol. 1995 Dec;22(12):2250-8. J Rheumatol. 1995. PMID: 8835558 - Reduced cartilage proteoglycan loss during zymosan-induced gonarthritis in NOS2-deficient mice and in anti-interleukin-1-treated wild-type mice with unabated joint inflammation.
van de Loo FA, Arntz OJ, van Enckevort FH, van Lent PL, van den Berg WB. van de Loo FA, et al. Arthritis Rheum. 1998 Apr;41(4):634-46. doi: 10.1002/1529-0131(199804)41:4<634::AID-ART10>3.0.CO;2-1. Arthritis Rheum. 1998. PMID: 9550472 - Adenoviral vector-mediated overexpression of IL-4 in the knee joint of mice with collagen-induced arthritis prevents cartilage destruction.
Lubberts E, Joosten LA, van Den Bersselaar L, Helsen MM, Bakker AC, van Meurs JB, Graham FL, Richards CD, van Den Berg WB. Lubberts E, et al. J Immunol. 1999 Oct 15;163(8):4546-56. J Immunol. 1999. PMID: 10510398 - Joint inflammation and cartilage destruction may occur uncoupled.
van den Berg WB. van den Berg WB. Springer Semin Immunopathol. 1998;20(1-2):149-64. doi: 10.1007/BF00832004. Springer Semin Immunopathol. 1998. PMID: 9836374 Review. - The role of cytokines in osteoarthritis pathophysiology.
Fernandes JC, Martel-Pelletier J, Pelletier JP. Fernandes JC, et al. Biorheology. 2002;39(1-2):237-46. Biorheology. 2002. PMID: 12082286 Review.
Cited by
- Osteoarthritic Synovial Fluid and TGF-β1 Induce Interleukin-18 in Articular Chondrocytes.
Carballo CB, Coelho TRP, de Holanda Afonso RC, Faria JCO, Alves T, Monte SM, Ventura Matioszek GM, Moura-Neto V, Brito JM. Carballo CB, et al. Cartilage. 2020 Jul;11(3):385-394. doi: 10.1177/1947603518796149. Epub 2018 Aug 27. Cartilage. 2020. PMID: 30146893 Free PMC article. - Early response of mouse joint tissue to noninvasive knee injury suggests treatment targets.
Wu P, Holguin N, Silva MJ, Fu M, Liao W, Sandell LJ. Wu P, et al. Arthritis Rheumatol. 2014 May;66(5):1256-65. doi: 10.1002/art.38375. Arthritis Rheumatol. 2014. PMID: 24470303 Free PMC article. - Therapeutic efficacy of intra-articular hyaluronan derivative and platelet-rich plasma in mice following axial tibial loading.
Duan X, Sandell LJ, Chinzei N, Holguin N, Silva MJ, Schiavinato A, Rai MF. Duan X, et al. PLoS One. 2017 Apr 13;12(4):e0175682. doi: 10.1371/journal.pone.0175682. eCollection 2017. PLoS One. 2017. PMID: 28406954 Free PMC article. - Cells of the synovium in rheumatoid arthritis. Chondrocytes.
Otero M, Goldring MB. Otero M, et al. Arthritis Res Ther. 2007;9(5):220. doi: 10.1186/ar2292. Arthritis Res Ther. 2007. PMID: 18001488 Free PMC article. Review. - Interplay of Oxidative Stress, Inflammation, and Autophagy in RAW 264.7 Murine Macrophage Cell Line Challenged with Si/SiO2 Quantum Dots.
Stanca L, Geicu OI, Serban AI, Dinischiotu A. Stanca L, et al. Materials (Basel). 2023 Jul 19;16(14):5083. doi: 10.3390/ma16145083. Materials (Basel). 2023. PMID: 37512357 Free PMC article.
References
- Ushio S, Namba M, Okura T, Hattori K, Nukada Y, Akita K, Tanabe F, Konishi K, Micallef M, Fujii M, Torigoe K, Tanimoto T, Fukuda S, Ikeda M, Okamura H, Kurimoto M. Cloning of the cDNA for human IFN-gamma-inducing factor, expression in Escherichia coli, and studies on the biologic activities of the protein. J Immunol. 1996;156:4274–4279. - PubMed
- Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okamoto I, Usui M, Ikeda M, Kurimoto M. IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–1550. - PubMed
- Gu Y, Kuida K, Tsutsui H, Ku G, Hsiao K, Fleming MA, Hayashi N, Higashino K, Okamura H, Nakanishi K, Kurimoto M, Tanimoto T, Flavell RA, Sato V, Harding MW, Livingston DJ, Su MS. Activation of interferon-gamma inducing factor mediated by interleukin-1beta converting enzyme. Science. 1997;275:206–209. - PubMed
- Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann NY Acad Sci. 1998;856:1–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous