A transmembrane protein required for acetylcholine receptor clustering in Caenorhabditis elegans - PubMed (original) (raw)
A transmembrane protein required for acetylcholine receptor clustering in Caenorhabditis elegans
Christelle Gally et al. Nature. 2004.
Abstract
Clustering neurotransmitter receptors at the synapse is crucial for efficient neurotransmission. Here we identify a Caenorhabditis elegans locus, lev-10, required for postsynaptic aggregation of ionotropic acetylcholine receptors (AChRs). lev-10 mutants were identified on the basis of weak resistance to the anthelminthic drug levamisole, a nematode-specific cholinergic agonist that activates AChRs present at neuromuscular junctions (NMJs) resulting in muscle hypercontraction and death at high concentrations. In lev-10 mutants, the density of levamisole-sensitive AChRs at NMJs is markedly reduced, yet the number of functional AChRs present at the muscle cell surface remains unchanged. LEV-10 is a transmembrane protein localized to cholinergic NMJs and required in body-wall muscles for AChR clustering. We also show that the LEV-10 extracellular region, containing five predicted CUB domains and one LDLa domain, is sufficient to rescue AChR aggregation in lev-10 mutants. This suggests a mechanism for AChR clustering that relies on extracellular protein-protein interactions. Such a mechanism is likely to be evolutionarily conserved because CUB/LDL transmembrane proteins similar to LEV-10, but lacking any assigned function, are expressed in the mammalian nervous system and might be used to cluster ionotropic receptors in vertebrates.
Conflict of interest statement
Competing interests statement The authors declare that they have no competing financial interests.
Figures
Figure 1
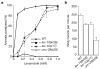
Phenotypic characterization of lev-10 mutants. a, The levamisole dose–response curve indicates that lev-10 mutants are only weakly resistant to levamisole when compared with unc-29(x29) mutants, which lack levamisole-sensitive AChRs. Error bars represent s.e.m. (n = 4 independent experiments). WT, wild type. b, lev-10 mutants exhibit weak locomotory defects compared with the wild type in a thrashing assay (ANOVA test; p < 0.01) but are not as impaired as unc-29(x29) mutants (p < 0.01). Error bars represent s.e.m. (n = 6).
Figure 2
Mutation of lev-10 results in the specific loss of levamisole-sensitive AChR clusters at neuromuscular junctions. a–d, UNC-29 localization detected by immunofluorescence with anti-UNC-29 antibodies. a, Shown are the nerve ring (nr) and the dorsal (dc) and ventral (vc) nerve cords in wild-type animals. c, Individual UNC-29 puncta at high magnification in the dorsal cord from the wild type. b, d, UNC-29 staining in lev-10(kr26) animals at magnifications as in a and c, respectively. The staining in the pharynx is non-specific (data not shown). e, Visualization of cholinergic varicosities by co-immunostaining of the vesicular ACh transporter UNC-17 in wild-type animals shows that UNC-29 clusters are juxtaposed to cholinergic release sites (arrowheads). f, UNC-17 staining in lev-10(kr26) mutants. g, h, Immunostaining of the GABA receptor UNC-49 in wild-type animals (g) and lev-10(kr26) mutants (h). Scale bars, 20 μm. i, Western blot with anti-UNC-29 and anti-VHA-5 antibodies on membrane fractions of C. elegans extracts. The UNC-29 protein has an apparent molecular mass of 47 kDa. VHA-5 detection is used for normalization. WT, wild type.
Figure 3
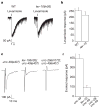
Levamisole-sensitive AChRs are functional but diffusely distributed in lev-10 body-wall muscle. a, Currents recorded from voltage-clamped body wall muscles in response to pressure-ejection of levamisole (300 μM) in wild-type (WT) and lev-10(kr26) mutants. b, Average amplitude of levamisole-elicited current. c, Evoked currents recorded in a body-wall muscle after eliciting neurotransmitter release by ventral nerve cord depolarization. Experiments were performed in an unc-49(e407) background to eliminate currents due to GABA receptor activation and in the presence of 5 μM DHβE, which blocks the levamisole-insensitive AChRs. d, Average amplitude of evoked response. Error bars in b and d represent s.e.m.
Figure 4
lev-10 encodes a CUB domain-rich transmembrane protein. a, Genomic organization of lev-10. Open boxes, coding regions; black boxes, 5′ and 3′ untranslated region; ATG, translational start site; SL1, _SL1 trans_-spliced leader. The first intron of lev-10 contains the first exon of the gene eat-18 (hatched boxes). The eat-18 exon is spliced to the second exon of lev-10 by using a different frame, which ends 16 bp after the splice site. ad1110, nonsense mutation in the first exon of eat-18. b, Predicted structure of the LEV-10 isoforms. Horizontal black line, signal peptide; CUB, complement, urchin epidermal growth factor, and bone morphogenetic protein domain; LDLa, low-density lipoprotein receptor domain class A; TM, transmembrane region; aa, amino acids. Domain predictions were based on SMART (smart.embl-heidelberg.de).
Figure 5
LEV-10 is a synaptic protein that requires levamisole-sensitive AChRs for proper localization but not for expression. a, LEV-10A immunostaining in the dorsal cord of a wild-type animal. c, UNC-17 immunostaining of the same animal labels cholinergic varicosities. e, Merged images. f, _Z_-optical projection through the entire stack of confocal images at the level of the dashed arrow in e. b, d, LEV-10A (b) and UNC-17 (d) immunostaining in the dorsal cord of an unc-29(x29) mutant. Scale bar, 10 μm. g, Western blot analysis of fractionated C. elegans extracts. P, pellet; S, cytosolic supernatant. The LEV-10 transmembrane protein has an apparent molecular mass of about 120 kDa. Mutants of the levamisole-sensitive AChR subunits unc-29(x29) and unc-38(x20) have LEV-10 concentrations at the membrane comparable to those of the wild type.
Similar articles
- A single immunoglobulin-domain protein required for clustering acetylcholine receptors in C. elegans.
Rapti G, Richmond J, Bessereau JL. Rapti G, et al. EMBO J. 2011 Feb 16;30(4):706-18. doi: 10.1038/emboj.2010.355. Epub 2011 Jan 21. EMBO J. 2011. PMID: 21252855 Free PMC article. - A secreted complement-control-related protein ensures acetylcholine receptor clustering.
Gendrel M, Rapti G, Richmond JE, Bessereau JL. Gendrel M, et al. Nature. 2009 Oct 15;461(7266):992-6. doi: 10.1038/nature08430. Epub 2009 Sep 30. Nature. 2009. PMID: 19794415 - Deciphering the molecular determinants of cholinergic anthelmintic sensitivity in nematodes: When novel functional validation approaches highlight major differences between the model Caenorhabditis elegans and parasitic species.
Blanchard A, Guégnard F, Charvet CL, Crisford A, Courtot E, Sauvé C, Harmache A, Duguet T, O'Connor V, Castagnone-Sereno P, Reaves B, Wolstenholme AJ, Beech RN, Holden-Dye L, Neveu C. Blanchard A, et al. PLoS Pathog. 2018 May 2;14(5):e1006996. doi: 10.1371/journal.ppat.1006996. eCollection 2018 May. PLoS Pathog. 2018. PMID: 29719008 Free PMC article. - Contributions from Caenorhabditis elegans functional genetics to antiparasitic drug target identification and validation: nicotinic acetylcholine receptors, a case study.
Brown LA, Jones AK, Buckingham SD, Mee CJ, Sattelle DB. Brown LA, et al. Int J Parasitol. 2006 May 31;36(6):617-24. doi: 10.1016/j.ijpara.2006.01.016. Epub 2006 Mar 6. Int J Parasitol. 2006. PMID: 16620825 Review. - Molecular regulation of postsynaptic differentiation at the neuromuscular junction.
Madhavan R, Peng HB. Madhavan R, et al. IUBMB Life. 2005 Nov;57(11):719-30. doi: 10.1080/15216540500338739. IUBMB Life. 2005. PMID: 16511964 Review.
Cited by
- From genes to function: the C. elegans genetic toolbox.
Boulin T, Hobert O. Boulin T, et al. Wiley Interdiscip Rev Dev Biol. 2012 Jan-Feb;1(1):114-37. doi: 10.1002/wdev.1. Epub 2011 Nov 28. Wiley Interdiscip Rev Dev Biol. 2012. PMID: 23801671 Free PMC article. Review. - Identification and characterization of novel nicotinic receptor-associated proteins in Caenorhabditis elegans.
Gottschalk A, Almedom RB, Schedletzky T, Anderson SD, Yates JR 3rd, Schafer WR. Gottschalk A, et al. EMBO J. 2005 Jul 20;24(14):2566-78. doi: 10.1038/sj.emboj.7600741. Epub 2005 Jun 30. EMBO J. 2005. PMID: 15990870 Free PMC article. - An extracellular scaffolding complex confers unusual rectification upon an ionotropic acetylcholine receptor in C. elegans.
Jospin M, Bonneau B, Lainé V, Bessereau JL. Jospin M, et al. Proc Natl Acad Sci U S A. 2022 Jul 19;119(29):e2113545119. doi: 10.1073/pnas.2113545119. Epub 2022 Jul 12. Proc Natl Acad Sci U S A. 2022. PMID: 35858330 Free PMC article. - UNC-108/RAB-2 and its effector RIC-19 are involved in dense core vesicle maturation in Caenorhabditis elegans.
Sumakovic M, Hegermann J, Luo L, Husson SJ, Schwarze K, Olendrowitz C, Schoofs L, Richmond J, Eimer S. Sumakovic M, et al. J Cell Biol. 2009 Sep 21;186(6):897-914. doi: 10.1083/jcb.200902096. J Cell Biol. 2009. PMID: 19797081 Free PMC article. - Eight genes are required for functional reconstitution of the Caenorhabditis elegans levamisole-sensitive acetylcholine receptor.
Boulin T, Gielen M, Richmond JE, Williams DC, Paoletti P, Bessereau JL. Boulin T, et al. Proc Natl Acad Sci U S A. 2008 Nov 25;105(47):18590-5. doi: 10.1073/pnas.0806933105. Epub 2008 Nov 19. Proc Natl Acad Sci U S A. 2008. PMID: 19020092 Free PMC article.
References
- Lewis JA, Wu CH, Levine JH, Berg H. Levamisole-resistant mutants of the nematode Caenorhabditis elegans appear to lack pharmacological acetylcholine receptors. Neuroscience. 1980;5:967–989. - PubMed
- Bessereau JL, et al. Mobilization of a Drosophila transposon in the Caenorhabditis elegans germ line. Nature. 2001;413:70–74. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases