Functional interdependence between septin and actin cytoskeleton - PubMed (original) (raw)
Functional interdependence between septin and actin cytoskeleton
Katja Schmidt et al. BMC Cell Biol. 2004.
Abstract
Background: Septin2 is a member of a highly conserved GTPase family found in fungi and animals. Septins have been implicated in a diversity of cellular processes including cytokinesis, formation of diffusion barriers and vesicle trafficking. Septin2 partially co-localises with actin bundles in mammalian interphase cells and Septin2-filamentmorphology depends upon an intact actin cytoskeleton. How this interaction is regulated is not known. Moreover, evidence that Septin2 is remodelled or redistributed in response to other changes in actin organisation is lacking.
Results: Septin2 filaments are associated with actin fibres, but Septin2 is not associated with actin at the leading edge of moving cells or in ruffles where actin is highly dynamic. Rather, Septin2 is spatially segregated from these active areas and forms O- and C-shaped structures, similar to those previously observed after latrunculin treatment. FRAP experiments showed that all assemblies formed by Septin2 are highly dynamic with a constant exchange of Septin2 in and out of these structures, and that this property is independent of actin. A combination of RNAi experiments and expression of truncated forms of Septin2 showed that Septin2 plays a significant role in stabilising or maintaining actin bundles.
Conclusion: We show that Septin2 can form dynamic structures with differing morphologies in living cells, and that these morphologies are dependent on the functional state of the actin cytoskeleton. Our data provide a link between the different morphological states of Septin2 and functions of Septin2 in actin-dynamics, and are consistent with the model proposed by Kinoshita and colleagues, that Septin2 filaments play a role in stabilisation of actin stress fibres thus preventing actin turnover.
Figures
Figure 1
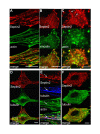
Septin2 interacts with actin, but not with tubulin. (A) Immunofluorescence of endogenous Septin2 (red) and actin (green, stained with phallacidin) in interphase NRK cells. Note the typical filamentous-granular organisation of Septin2 and its co-localisation with actin. (B) Septin2 (red) does not colocalise with endogenous vinculin (green), a marker for focal adhesions. (C) Staining of Septin2 (red) and actin (green) after disruption of the actin cytoskeleton. NRK cells were treated with Latrunculin B for 30 min. Septin2 now forms rings. (D) Septin2 (red) and tubulin (green) mainly have distinct distributions in NRK cells. (E) Enlarged image of the boxed region in D. Regions with overlapping Septin2 (red) and tubulin (blue) staining always contain actin (green) as well (arrow). (F) Cells were treated with nocodazole for 30 min. Microtubule organisation (green) is disturbed, but Septin2 (red) distribution is largely unaffected. All images show a single confocal section. Bars, 10 μm.
Figure 2
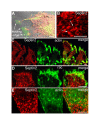
Knock down of Septin2 expression abolishes actin organisation. NRK cells were transfected with siRNA targeting Septin2. (A) Immunofluorescence 48 hrs after transfection. Cells with reduced level of Septin2 (red) have no actin bundles (red) (0). (B) Tubulin distribution (green) is unaffected in cells showing a decreased Septin2 staining (red) (0). (C) 48 hrs after transfection with siRNA NRK cells were lysed and immunoblotted for Septin2, actin, tubulin and Erp72. The amounts of Septin2, actin and tubulin were quantified and normalized to the corresponding amount of Erp72 to account for reduction in cell number upon siRNA treatment. co, control; Erp72, Endoplasmic Reticulum Protein, member of the protein disulfide isomerase family. Similar results were obtained with Hela cells. A single confocal section is shown. Bars, 20 μm.
Figure 3
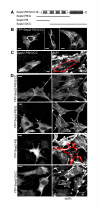
Characterisation of truncated YFP-Septin2 constructs. (A) Schematic presentation of full length YFP-Septin2 (YFP-Sept2-PB/G/CC) and truncated YFP-Septin2 constructs. (B) In some cells overexpression of full length YFP-Sept2-PB/G/CC induced the formation of higher-ordered YFP-Septin2 containing-structures with differing morphology (C) Untagged full length Sept2-PB/G/CC is also capable of forming these structures and actin filaments are attenuated in those cells. Full length Sept2-PB/G/CC was detected with anti-Septin2-antibody. (D) Distribution of YFP-Septin2 constructs in NRK cells and their effect on the actin cytoskeleton. Cells were transfected with either full length YFP-Sept2-PB/G/CC or the truncated YFP-Septin2 constructs and stained for actin with phallacidin. Only YFP-Sept2-PB, the construct lacking the GTPase domain and the coiled coil domain affects actin organisation. The distributions of YFP-Sept2-PB/G, the construct without the coiled-coil domain, and YFP-Sept2-G/CC, which lacks the polybasic region, are different to full length YFP-Sept2-PB/G/CC, but there is no effect on actin. Images show a single confocal section. Bars. 10 μm.
Figure 4
Effects of Rho-GTPases on actin and Septin2 organisation. NRK cells were transfected with GDP-locked forms of myc-Rac1 (A), myc-CDC42 (B) or myc-RhoA (C) and stained for Septin2 and actin. GTPases were detected with a monoclonal anti myc-antibody. (A) Cells expressing the myc-Rac1 mutant lack actin bundles and Septin2 organisation is disrupted. Instead of filaments, Septin2 forms rings (inset, inset was taken from another cell transfected with Rac1-mutant). (B) myc-CDC42-GDP induces the formation of thick actin bundles but has only little effect on Septin2 appearance. (C) Transfection with dominant-negativ myc-RhoA causes the detachment of most of the cells. Cells left on the coverslip have diminished actin filaments and disrupted Septin2 organisation. Images are single confocal sections. Bars, 10 μm.
Figure 5
Septin2 is not associated with actin in moving or ruffling cells. (A) DIC image of moving NRK cells in an experimentally wounded monolayer. Note the leading edges of the cells contain actin (green), but Septin2 (red) is completely missing. (B) Septin2 forms ring-like structures in the body of moving cells comparable to rings observed after latrunculin treatment (compare Fig. 1C). (C) In ruffling cells growing on lysine-coated coverslips Septin2 (red) is detectable in the cell body, whereas actin (green) is concentrated in ruffles. (D) Ruffles are also positive for endogenous Rac1 (green), but Septin2 (red) is clearly missing. (E) In the cell body of ruffling cells Septin2 (red) forms O- and C-shaped ring-like structures, which are not associated with actin (green, stained with phallacidin). Bars, 5 μm.
Figure 6
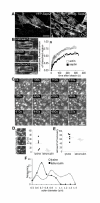
Septin2 forms highly dynamic filaments and rings. (A) YFP-Septin2 has the same distribution like endogenous Septin2 and it does not alter the organisation of endogenous Septin2. Bar, 20 μm (B) Photobleach experiment. NRK cells were transfected with either YFP-Septin2 or GFP-actin. Part of the filaments was bleached and recovery monitored over time. The ratio between mean fluorescence intensity of the prebleached box and the mean fluorescence of the whole cell was normalised to the prebleach ratio and expressed as a function of time (Materials and Methods). The graph shows representative recovery curves, which were fitted to a single exponential curve (solid lines) to calculate tDs and amount of recovery. n = 5. Images show a single confocal section of YFP-Septin2 filaments in NRK cells. Bar, 4 μm. (C) Images of a time-laps movie of YFP-Septin2 in the cell body of ruffling NRK-cells. Arrows indicate the formation cycle of a ring. Bar, 2 μm. (D,E) Photobleach experiments of YFP-Septin2 rings (also see additional file 1 for lysine/ruffling cells and additional file 2 for latrunculin). NRK cells growing on lysine-coated coverslips to induce ruffles were transfected with YFP-Septin2. Rings formed in the cell body were bleached and recovery monitored over time. For bleaching of Septin2 rings upon latrunculin treatment, normal NRK cells were transfected with YFP-Septin2 and treated with Latrunculin B for 20–25 Min before photobleaching. The data were analysed as in B to calculate tDs of recovery (D) and amount of recovery (E). n = 5. Images show a single confocal section of YFP-Septin2 rings in ruffling NRK cells. (F) Quantitative comparison of outer diameters of Septin2 rings in ruffling cells (lysine, empty circles) and upon latrunculin treatment (filled circles). Measurements were done on images of cells transfected with YFP-Septin2.
Similar articles
- Rho GTPases: molecular switches that control the organization and dynamics of the actin cytoskeleton.
Hall A, Nobes CD. Hall A, et al. Philos Trans R Soc Lond B Biol Sci. 2000 Jul 29;355(1399):965-70. doi: 10.1098/rstb.2000.0632. Philos Trans R Soc Lond B Biol Sci. 2000. PMID: 11128990 Free PMC article. Review. - The pathobiology of the septin gene family.
Hall PA, Russell SE. Hall PA, et al. J Pathol. 2004 Nov;204(4):489-505. doi: 10.1002/path.1654. J Pathol. 2004. PMID: 15495264 Review. - Reorganisation of the dendritic actin network during cancer cell migration and invasion.
Vignjevic D, Montagnac G. Vignjevic D, et al. Semin Cancer Biol. 2008 Feb;18(1):12-22. doi: 10.1016/j.semcancer.2007.08.001. Epub 2007 Sep 4. Semin Cancer Biol. 2008. PMID: 17928234 Review. - Mechanisms of actin filament turnover in animal cells.
Sheterline P. Sheterline P. Symp Soc Exp Biol. 1993;47:339-52. Symp Soc Exp Biol. 1993. PMID: 8165575 Review. - Distribution of profilin in fibroblasts correlates with the presence of highly dynamic actin filaments.
Buss F, Temm-Grove C, Henning S, Jockusch BM. Buss F, et al. Cell Motil Cytoskeleton. 1992;22(1):51-61. doi: 10.1002/cm.970220106. Cell Motil Cytoskeleton. 1992. PMID: 1581979
Cited by
- Reversible paralysis of Schistosoma mansoni by forchlorfenuron, a phenylurea cytokinin that affects septins.
Zeraik AE, Galkin VE, Rinaldi G, Garratt RC, Smout MJ, Loukas A, Mann VH, Araujo AP, DeMarco R, Brindley PJ. Zeraik AE, et al. Int J Parasitol. 2014 Jul;44(8):523-31. doi: 10.1016/j.ijpara.2014.03.010. Epub 2014 Apr 21. Int J Parasitol. 2014. PMID: 24768753 Free PMC article. - Microtubules support a disk-like septin arrangement at the plasma membrane of mammalian cells.
Sellin ME, Holmfeldt P, Stenmark S, Gullberg M. Sellin ME, et al. Mol Biol Cell. 2011 Dec;22(23):4588-601. doi: 10.1091/mbc.E11-09-0754. Epub 2011 Oct 12. Mol Biol Cell. 2011. PMID: 21998205 Free PMC article. - The evolution, complex structures and function of septin proteins.
Cao L, Yu W, Wu Y, Yu L. Cao L, et al. Cell Mol Life Sci. 2009 Oct;66(20):3309-23. doi: 10.1007/s00018-009-0087-2. Epub 2009 Jul 14. Cell Mol Life Sci. 2009. PMID: 19597764 Free PMC article. Review. - A homozygous genome-edited Sept2-EGFP fibroblast cell line.
Banko M, Mucha-Kruczynska I, Weise C, Heyd F, Ewers H. Banko M, et al. Cytoskeleton (Hoboken). 2019 Jan;76(1):73-82. doi: 10.1002/cm.21518. Epub 2019 Apr 19. Cytoskeleton (Hoboken). 2019. PMID: 30924304 Free PMC article. - ACTN4 Mediates SEPT14 Mutation-Induced Sperm Head Defects.
Lin YH, Huang CY, Ke CC, Wang YY, Lai TH, Liu HC, Ku WC, Chan CC, Lin YH. Lin YH, et al. Biomedicines. 2020 Nov 19;8(11):518. doi: 10.3390/biomedicines8110518. Biomedicines. 2020. PMID: 33228246 Free PMC article.
References
- Kartmann B, Roth D. Novel roles for mammalian septins: from vesicle trafficking to oncogenesis. J Cell Sci. 2001;114:839–844. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous