Condensin restructures chromosomes in preparation for meiotic divisions - PubMed (original) (raw)
Condensin restructures chromosomes in preparation for meiotic divisions
Raymond C Chan et al. J Cell Biol. 2004.
Abstract
The production of haploid gametes from diploid germ cells requires two rounds of meiotic chromosome segregation after one round of replication. Accurate meiotic chromosome segregation involves the remodeling of each pair of homologous chromosomes around the site of crossover into a highly condensed and ordered structure. We showed that condensin, the protein complex needed for mitotic chromosome compaction, restructures chromosomes during meiosis in Caenorhabditis elegans. In particular, condensin promotes both meiotic chromosome condensation after crossover recombination and the remodeling of sister chromatids. Condensin helps resolve cohesin-independent linkages between sister chromatids and alleviates recombination-independent linkages between homologues. The safeguarding of chromosome resolution by condensin permits chromosome segregation and is crucial for the formation of discrete, individualized bivalent chromosomes.
Figures
Figure 1.
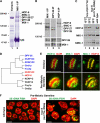
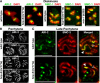
HCP-6 associates exclusively with the mitotic condensin II complex and colocalizes with MIX-1 on mitotic chromosomes. (A) Coomassie staining and microsequencing identified proteins in MIX-1 IPs. (B) Western blot analysis of MIX-1, DPY-28, and HCP-6 IPs confirmed association of DPY-28 and HCP-6 with MIX-1. Dosage compensation proteins DPY-26 and DPY-27 were found only in DPY-28 and MIX-1 IPs (lane 2), and mitotic condensin subunit SMC-4 only in HCP-6 and MIX-1 IPs (lane 3). Blots were probed with mixtures of antibodies. (C) HCP-6 protein levels were not reduced in hcp-6(mr17) mutants relative to the MIX-1 and SMC-1 loading controls, but HCP-6 was undetectable in hcp-6(mr17, RNAi) mutants. (D) Phylogenetic tree comparing DPY-28, HCP-6, and CAP-D2, CAP-D3, and CAP-G homologues. HCP-6 is closest to CAP-D3 of condensin II. (E) HCP-6 and MIX-1 colocalized on metaphase chromosomes in embryos and the premeiotic germline. (F) 5S rDNA FISH revealed aneuploid nuclei in the premeiotic germline of hcp-6 mutant hermaphrodites. Bars, 5 μm.
Figure 2.
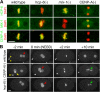
HCP-6 and MIX-1 associate independently with mitotic chromosomes and are required for chromosome condensation and segregation. (A) HCP-6 was undetectable in hcp-6(mr17, RNAi) mutant embryos, yet MIX-1 and CENP-A still associated with chromosomes at the disorganized metaphase plate. Similarly, MIX-1 was undetectable in mix-1(b285, RNAi) embryos, but HCP-6 and CENP-A still accumulated on chromosomes. HCP-6 did not associate with chromosomes of embryos depleted for two CENP-A paralogues, but MIX-1 did. (B) Mitotic chromosomes tagged with GFP::H2B histone condensed in pronuclei of wild-type embryos (red arrowhead) at least 2 min before NEBD, but decondensed chromosomes persisted until NEBD in pronuclei of hcp-6(mr17) and CENP-A–depleted embryos (red arrows). Defective mitotic chromosome segregation resulted in the formation of anaphase bridges (green arrows). Two polar bodies were extruded during meiosis in wild-type embryos (yellow arrowheads), but extra pronuclei formed in CENP-A–depleted embryos (yellow arrow) due to meiotic defects. Bars, 5 μm.
Figure 3.
HCP-6 is required for chromosome segregation in meiosis I and II. In wild-type zygotes, GFP::H2B histone-tagged chromosomes align on the metaphase plate after breakdown of the oocyte nuclear envelope. Homologues separate in anaphase of meiosis I; one set is extruded into the first polar body (PB1). Sister chromatids separate in meiosis II; one set is extruded into the second polar body (PB2). The second set decondenses and forms the oocyte pronucleus (O). In hcp-6(mr17) and hcp-6(mr17, RNAi) mutants, DNA bridges connected separating chromosomes in anaphase I and II (arrows). Bars, 5 μm.
Figure 4.
Homologues undergo dramatic structural reorganization during prophase of meiosis I. This diagram depicts the progression of a pair of homologues through prophase I. Homologue pairing and alignment initiate in leptotene–zygotene (not depicted). In pachytene, SC (yellow line) stabilizes homologue synapsis (red and green lines). A single crossover typically divides each homologue pair into long and short segments. SC disassembly in diplotene results first in desynapsis of the long segment of each homologue pair and then of the short segment (Nabeshima, K., M. Colaiácovo, and A. Villeneuve, personal communication). Recombinant homologues are reorganized around the crossover site and condensed to form highly compacted, cruciform bivalents. The short segment becomes the short arm of the diakinesis bivalent, where cohesin-mediated linkages maintain the association of homologues. Importantly, HCP-6 and MIX-1 (blue dots) become enriched on chromosomes after pachytene exit, and our evidence indicates that both proteins are required for the reorganization of homologues and formation of discrete, compacted diakinesis bivalents.
Figure 5.
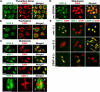
HCP-6 and MIX-1 first associate with meiotic chromosomes at diplotene–diakinesis. In confocal images of wild-type transition zone (A) and pachytene (B) nuclei, HCP-6 appeared nucleoplasmic but excluded from DNA (arrowheads indicate regions devoid of HCP-6 staining). (C) HCP-6 and MIX-1 accumulate in four quadrants on wild-type diakinesis bivalents. CENP-A was also present in four foci per bivalent, but these foci were broader than the HCP-6 and MIX-1 foci. The four HCP-6 foci (fourth row, arrowheads) were bisected by SMC-1 staining, indicating that each quadrant represents one sister chromatid. (D) After partial depletion of REC-8, two HCP-6 foci (arrowheads) were present on each univalent, correlating with the presence of two sister chromatids per univalent. (E) MIX-1 was undetectable on bivalents in mix-1(b285, RNAi) mutants, but HCP-6 still accumulated on chromosomes. In contrast, MIX-1 required HCP-6 for its association with meiotic chromosomes. MIX-1 was undetectable on bivalents in animal mutants for the partial loss-of-function allele hcp-6(mr17), which also disrupted HCP-6 loading. Association of MIX-1 with the dosage compensation complex does not require HCP-6: MIX-1 antibodies stained X chromosomes in gut nuclei of hcp-6(mr17) and hcp-6(mr17, RNAi) mutants (not depicted). Both MIX-1 and HCP-6 loaded on meiotic chromosomes in CENP-A depleted worms, and CENP-A localized on chromosomes in mix-1(b285, RNAi) and hcp-6(mr17, RNAi) oocytes. Bars, 2 μm.
Figure 6.
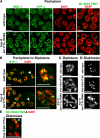
HCP-6 mediates chromosome compaction and resolution at diplotene–diakinesis. (A) Confocal images of DAPI staining and 5S rDNA FISH revealed normal DNA morphology and homologue association in pachytene of hcp-6 mutants. Moreover, staining of cohesin subunit SMC-1 and SC central element SYP-1 were indistinguishable in wild-type and hcp-6 mutant pachytene nuclei. (B) Residual SYP-1 staining in late pachytene and diplotene nuclei was similar in wild-type and hcp-6(mr17, RNAi) animals (left column, arrowhead), suggesting normal SC disassembly in hcp-6 mutants. However, wild-type chromosomes compacted rapidly in diplotene but hcp-6 mutant chromosomes were decondensed (right column, magnified images of nuclei marked with arrowheads in left column). (C) Diplotene chromosomes decondensed in hcp-6(mr17, RNAi) and hcp-6(mr17) mutants. (D) Resolution of diakinesis bivalents failed in hcp-6(mr17, RNAi) animals. Six compact bivalents were resolved by late diakinesis in hcp-6(mr17) mutants, as in wild type (see Fig. 8 E). (E) 5S rDNA FISH indicated that homologue realignment of chromosome V (arrowhead) was achieved by diakinesis in hcp-6(mr17) mutants. Bars, 5 μm.
Figure 7.
Condensin is required for chromosome organization within diakinesis bivalents. (A) AIR-2 localized at the midbivalent in wild-type and hcp-6(mr17) animals. In contrast, SMC-1 adopted a cruciform pattern in wild-type bivalents, but was disorganized in hcp-6(mr17) and mix-1(b285) bivalents. Each panel shows a representative subset of bivalents in a single diakinesis nucleus. (B) AIR-2 and SMC-3 colocalized along the length of synapsed pachytene homologues in wild-type and hcp-6(mr17) animals. (C) During SC disassembly, AIR-2 diminished along the desynapsed region of each homologue pair. AIR-2 persisted along the synapsed region (solid arrowhead). SMC-3 staining persisted along the entire length of homologue pairs, revealing the separation of desynapsed regions (hollow arrowhead). Bars, 5 μm.
Figure 8.
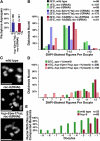
Condensin mutations blocked the resolution of cohesin-independent linkages between sister chromatids and between homologues in diplotene–diakinesis. (A) 5S rDNA FISH showed equivalent homologue asynapsis in hcp-6(mr17);rec-8(RNAi) double mutants at 15 and 25°C. (B) Both hcp-6(mr17) and mix-1(b285) blocked precocious homologue and sister chromatid separation caused by rec-8(RNAi). Wild-type diakinesis nuclei contain six bivalents. 7–12 DAPI-staining bodies indicate separation of one or more homologue pairs; more than 12 DAPI-staining bodies indicates separation of both sister chromatids and homologues. n = number of oocytes scored. (C) The number and size of DAPI-staining bodies of wild-type, rec-8(RNAi), and hcp-6(mr17);rec-8(RNAi) animals showed that hcp-6(mr17) prevents premature homologue separation. The arrowhead marks a normal bivalent in a rec-8(RNAi) animal. Bars, 5 μm. (D) The hcp-6(mr17) mutation prevents homologue separation in spo-11(me44) animals. Similar phenotypes resulted from spo-11(me44) at 15 and 25°C (P = 1; Table S3, available at
http://www.jcb.org/cgi/content/full/jcb.200408061/DC1
). n = number of oocytes scored. (E) Resolution of diakinesis bivalents is delayed in hcp-6(mr17) mutants relative to wild type. The percentage of oocytes with any resolved bivalents is plotted against oocyte position in the gonad, which reflects the age of the oocyte (−9 oocyte, early diplotene; −1 oocyte, oldest oocyte). n = number of gonad arms scored.
Similar articles
- Condensin I protects meiotic cohesin from WAPL-1 mediated removal.
Hernandez MR, Davis MB, Jiang J, Brouhard EA, Severson AF, Csankovszki G. Hernandez MR, et al. PLoS Genet. 2018 May 16;14(5):e1007382. doi: 10.1371/journal.pgen.1007382. eCollection 2018 May. PLoS Genet. 2018. PMID: 29768402 Free PMC article. - C. elegans condensin promotes mitotic chromosome architecture, centromere organization, and sister chromatid segregation during mitosis and meiosis.
Hagstrom KA, Holmes VF, Cozzarelli NR, Meyer BJ. Hagstrom KA, et al. Genes Dev. 2002 Mar 15;16(6):729-42. doi: 10.1101/gad.968302. Genes Dev. 2002. PMID: 11914278 Free PMC article. - The aurora B kinase AIR-2 regulates kinetochores during mitosis and is required for separation of homologous Chromosomes during meiosis.
Kaitna S, Pasierbek P, Jantsch M, Loidl J, Glotzer M. Kaitna S, et al. Curr Biol. 2002 May 14;12(10):798-812. doi: 10.1016/s0960-9822(02)00820-5. Curr Biol. 2002. PMID: 12015116 - Meiosis.
Hillers KJ, Jantsch V, Martinez-Perez E, Yanowitz JL. Hillers KJ, et al. WormBook. 2017 May 4;2017:1-43. doi: 10.1895/wormbook.1.178.1. WormBook. 2017. PMID: 26694509 Free PMC article. Review. - Condensins: organizing and segregating the genome.
Hirano T. Hirano T. Curr Biol. 2005 Apr 12;15(7):R265-75. doi: 10.1016/j.cub.2005.03.037. Curr Biol. 2005. PMID: 15823530 Review.
Cited by
- akirin is required for diakinesis bivalent structure and synaptonemal complex disassembly at meiotic prophase I.
Clemons AM, Brockway HM, Yin Y, Kasinathan B, Butterfield YS, Jones SJ, Colaiácovo MP, Smolikove S. Clemons AM, et al. Mol Biol Cell. 2013 Apr;24(7):1053-67. doi: 10.1091/mbc.E12-11-0841. Epub 2013 Jan 30. Mol Biol Cell. 2013. PMID: 23363597 Free PMC article. - HCP-4/CENP-C promotes the prophase timing of centromere resolution by enabling the centromere association of HCP-6 in Caenorhabditis elegans.
Moore LL, Stanvitch G, Roth MB, Rosen D. Moore LL, et al. Mol Cell Biol. 2005 Apr;25(7):2583-92. doi: 10.1128/MCB.25.7.2583-2592.2005. Mol Cell Biol. 2005. PMID: 15767665 Free PMC article. - xnd-1 regulates the global recombination landscape in Caenorhabditis elegans.
Wagner CR, Kuervers L, Baillie DL, Yanowitz JL. Wagner CR, et al. Nature. 2010 Oct 14;467(7317):839-43. doi: 10.1038/nature09429. Nature. 2010. PMID: 20944745 Free PMC article. - Condensin and cohesin complexity: the expanding repertoire of functions.
Wood AJ, Severson AF, Meyer BJ. Wood AJ, et al. Nat Rev Genet. 2010 Jun;11(6):391-404. doi: 10.1038/nrg2794. Epub 2010 May 5. Nat Rev Genet. 2010. PMID: 20442714 Free PMC article. Review.
References
- Adachi, J., and M. Hasegawa. 1996. MOLPHY version 2.3: programs for molecular phylogenetics based on maximum likelihood. Comput. Sci. Monogr. 28:1–150.
- Agresti, A. 1992. A survey of exact inference for contingency tables. Stat. Sci. 7:131–153.
- Albertson, D.G., A.M. Rose, and A.M. Villeneuve. 1997. Chromosome organization, mitosis, and meiosis. C. elegans II. D.L. Riddle, T. Blumenthal, B.J. Meyer, and J.R. Priess, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 47–78. - PubMed
- Buchwitz, B.J., K. Ahmad, L.L. Moore, M.B. Roth, and S. Henikoff. 1999. A histone-H3-like protein in C. elegans. Nature. 401:547–548. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases