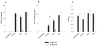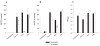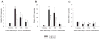Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia - PubMed (original) (raw)
Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia
Tammy Kielian et al. Glia. 2005 Mar.
Abstract
Toll-like receptor 2 (TLR2) is a pattern recognition receptor that plays an important role in enabling cells of the innate immune system to recognize conserved structural motifs on a wide array of pathogens including gram-positive bacteria. Although microglia have recently been shown to express TLR2, the functional significance of this receptor in mediating microglial activation remains unknown. To ascertain the importance of TLR2 in microglial responses to S. aureus and its cell wall product peptidoglycan (PGN), we evaluated primary microglia from TLR2 knockout (KO) and wild-type (WT) mice. TLR2 was found to play a pivotal role in PGN recognition and subsequent activation in primary microglia, as demonstrated by the attenuated expression of TNF-alpha, IL-12 p40, MIP-2, and MCP-1 in PGN-treated TLR2 KO microglia compared with WT cells. In contrast, the responses of TLR2 KO and WT microglia to S. aureus were qualitatively similar, indicating that alternative receptors are responsible for recognizing intact bacteria. Microarray analysis confirmed that TLR2 plays a central role in PGN recognition by primary microglia. The expression of MyD88, a central adapter molecule in TLR-dependent signaling, was similar in both TLR2 KO and WT microglia, suggesting that the defect in PGN recognition by the former is not due to alterations in this key signaling intermediate. These findings reveal the complex nature of gram-positive bacterial recognition by microglia, which occurs, in part, through engagement of TLR2.
Figures
Fig. 1
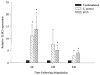
S. aureus and peptidoglycan (PGN) augment TLR2 mRNA expression in primary microglia. The time course profiles of TLR2 mRNA expression following S. aureus or PGN stimulation in primary wild-type microglia were measured by quantitative real-time RT-PCR as described in the Materials and Methods. Each real-time PCR reaction was performed in duplicate for both the target (TLR2) and the “housekeeping” gene GAPDH. The level of gene expression was calculated after normalizing TLR2 signals against GADPH and is presented in relative mRNA expression units (mean ± SD of four independent experiments). Significant differences between untreated versus S. aureus or PGN-stimulated microglia are denoted with asterisks (*P < 0.05).
Fig. 2
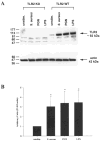
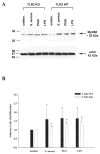
Both S. aureus and peptidoglycan (PGN) enhance TLR2 protein expression in primary microglia. TLR2 knockout (KO) and wild-type (WT) primary microglia were stimulated with either 107 heat-inactivated S. aureus, 10 μg/ml PGN, or 100 ng/ml LPS. Protein extracts from whole cell lysates were prepared 24 h following stimulation and evaluated for TLR2 expression by Western blotting as described in the Materials and Methods. Results are presented as the raw gel data (A) and quantitative analysis of TLR2 expression by densometric scanning (B). For quantitation in B, the pixel intensity of each TLR2 band from WT microglia was normalized to the amount of actin included as a “housekeeping” gene. Results are expressed in arbitrary units as the ratio of TLR2 to actin and represent the mean ± SD of three independent experiments. Significant differences are denoted with asterisks (*P < 0.05).
Fig. 3
TLR2 plays a pivotal role in peptidoglycan (PGN) recognition by primary microglia and subsequent proinflammatory cytokine production. TLR2 knockout (KO) and wild-type (WT) microglia were seeded at 2 × 105 cells per well in 96-well plates and incubated overnight. The following day, cells were exposed to 107 heat-inactivated S. aureus, 10 μg/ml PGN, or 100 ng/ml LPS for 24 h. Conditioned supernatants were collected and analyzed for TNF-α (A) and IL-12 p40 (B) by ELISA. Results are presented as the amount of cytokine (ng) per ml of culture supernatant (mean ± SD). Microglial cell viability was assessed using a standard MTT assay and the raw OD570 absorbance values are reported (mean ± SD, C). Significant differences between TLR2 WT and KO microglia are denoted with asterisks (**P < 0.001). Results are representative of four independent experiments.
Fig. 4
TLR2 KO microglia display defects in chemokine expression following stimulation with either S. aureus or peptidoglycan (PGN). Primary microglia from TLR2 knockout (KO) and wild-type (WT) mice were seeded at 2 × 105 cells per well in 96-well plates and incubated overnight. The following day, cells were exposed to 107 heat-inactivated S. aureus, 10 μg/ml PGN, or 100 ng/ml LPS for 24 h. Conditioned supernatants were collected and analyzed for MIP-2 (A) and MCP-1 (B) by ELISA. Results are presented as the amount of chemokine (ng) per ml of culture supernatant (mean ± SD). Microglial cell viability was assessed using a standard MTT assay and the raw OD570 absorbance values are reported (mean ± SD, C). Significant differences between TLR2 WT and KO microglia are denoted with asterisks (*P < 0.05, **P < 0.001). Results are representative of four independent experiments.
Fig. 5
MyD88 expression is increased in primary microglia following S. aureus and peptidoglycan (PGN) exposure. TLR2 knockout (KO) and wild-type (WT) primary microglia were stimulated with either 107 heat-inactivated S. aureus, 10 μg/ml PGN, or 100 ng/ml LPS. Protein extracts from whole cell lysates were prepared 24 h following stimulation and evaluated for MyD88 expression by Western blotting as described in the Materials and Methods. Results are presented as the raw gel data (A) and quantitative analysis of MyD88 expression by densometric scanning (B). For quantitation in B, the pixel intensity of each MyD88 band was normalized to the amount of actin included as a “housekeeping” gene. Results are expressed in arbitrary units as the ratio of MyD88 to actin and represent the mean ± SD of three independent experiments. Significant differences between unstimulated versus _S. aureus_-, PGN-, or LPS-treated microglia are denoted with asterisks (*P < 0.05).
Fig. 6
S. aureus increases the expression of additional receptors known to bind bacteria in both TLR2 knockout (KO) and wild-type (WT) microglia. The time course profiles of CD14 (A), LOX-1 (B), and MRC-1 (C) mRNA expression following S. aureus stimulation in both TLR2 KO and WT microglia were measured by quantitative real-time RT-PCR as described in the Materials and Methods. Each real-time PCR reaction was performed in duplicate for both the target (CD14, LOX-1, and MRC-1) and the “housekeeping” gene GAPDH. The level of gene expression was calculated after normalizing target signals against GADPH and is presented in relative mRNA expression units (mean ± SD of three independent experiments). Significant differences between untreated versus _S. aureus_-stimulated microglia are denoted with asterisks (*P < 0.05, **P < 0.001), whereas significant differences between TLR2 WT and KO microglia are also indicated (# P < 0.05).
Fig. 7
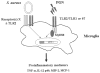
Pathways for recognition of intact S. aureus versus peptidoglycan (PGN) by microglia. Microglia primarily use TLR2 (hatched bars) for PGN recognition and subsequent proinflammatory mediator expression. It is currently unknown whether TLR2 heterodimerizes with TLR1 or TLR6 to facilitate PGN recognition by microglia. In contrast, alternative receptor(s) (denoted here as receptor(s) X) are responsible for mediating microglial activation in response to intact bacteria. Under certain circumstances, these receptor(s) may signal through cooperative interactions with TLR2. It is likely that intact S. aureus engages multiple cell surface receptors on microglia to ensure efficient innate immune responses within the CNS parenchyma.
Similar articles
- Central role for MyD88 in the responses of microglia to pathogen-associated molecular patterns.
Esen N, Kielian T. Esen N, et al. J Immunol. 2006 Jun 1;176(11):6802-11. doi: 10.4049/jimmunol.176.11.6802. J Immunol. 2006. PMID: 16709840 Free PMC article. - Recognition of Staphylococcus aureus-derived peptidoglycan (PGN) but not intact bacteria is mediated by CD14 in microglia.
Esen N, Kielian T. Esen N, et al. J Neuroimmunol. 2005 Dec 30;170(1-2):93-104. doi: 10.1016/j.jneuroim.2005.09.003. Epub 2005 Oct 17. J Neuroimmunol. 2005. PMID: 16229899 Free PMC article. - Toll-like receptor 2 (TLR2)-TLR9 crosstalk dictates IL-12 family cytokine production in microglia.
Holley MM, Zhang Y, Lehrmann E, Wood WH, Becker KG, Kielian T. Holley MM, et al. Glia. 2012 Jan;60(1):29-42. doi: 10.1002/glia.21243. Epub 2011 Sep 7. Glia. 2012. PMID: 21901759 Free PMC article. - Toll-like receptors; their physiological role and signal transduction system.
Takeuchi O, Akira S. Takeuchi O, et al. Int Immunopharmacol. 2001 Apr;1(4):625-35. doi: 10.1016/s1567-5769(01)00010-8. Int Immunopharmacol. 2001. PMID: 11357875 Review. - Toll-like receptors and their signaling mechanism in innate immunity.
Kaisho T, Akira S. Kaisho T, et al. Acta Odontol Scand. 2001 Jun;59(3):124-30. doi: 10.1080/000163501750266701. Acta Odontol Scand. 2001. PMID: 11501880 Review.
Cited by
- Activation of toll-like receptor signaling pathways leading to nitric oxide-mediated antiviral responses.
Abdul-Cader MS, Amarasinghe A, Abdul-Careem MF. Abdul-Cader MS, et al. Arch Virol. 2016 Aug;161(8):2075-86. doi: 10.1007/s00705-016-2904-x. Epub 2016 May 27. Arch Virol. 2016. PMID: 27233799 Free PMC article. Review. - Myricetin Inhibition of Peptidoglycan-Induced COX-2 Expression in H9c2 Cardiomyocytes.
Rosas-Martínez M, Gutiérrez-Venegas G. Rosas-Martínez M, et al. Prev Nutr Food Sci. 2019 Jun;24(2):202-209. doi: 10.3746/pnf.2019.24.2.202. Epub 2019 Jun 30. Prev Nutr Food Sci. 2019. PMID: 31328126 Free PMC article. - Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential.
Hanke ML, Kielian T. Hanke ML, et al. Clin Sci (Lond). 2011 Nov;121(9):367-87. doi: 10.1042/CS20110164. Clin Sci (Lond). 2011. PMID: 21745188 Free PMC article. Review. - Thickening of the walls of deep brain abscesses is associated with macrophage infiltration.
Yang Z, Yang Y, Qi X, Liu N, Wang P, Zhang L, Han M, Han S. Yang Z, et al. Exp Ther Med. 2021 Oct;22(4):1080. doi: 10.3892/etm.2021.10514. Epub 2021 Jul 29. Exp Ther Med. 2021. PMID: 34447473 Free PMC article. - Central role for MyD88 in the responses of microglia to pathogen-associated molecular patterns.
Esen N, Kielian T. Esen N, et al. J Immunol. 2006 Jun 1;176(11):6802-11. doi: 10.4049/jimmunol.176.11.6802. J Immunol. 2006. PMID: 16709840 Free PMC article.
References
- Akira S. Toll-like receptor signaling. J Biol Chem. 2003;278:38105–38108. - PubMed
- Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. - PubMed
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. - PubMed
- Dalpke AH, Schafer MK, Frey M, Zimmermann S, Tebbe J, Weihe E, Heeg K. Immunostimulatory CpG-DNA activates murine microglia. J Immunol. 2002;168:4854–4863. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH065297/MH/NIMH NIH HHS/United States
- R01 MH65297L/MH/NIMH NIH HHS/United States
- P20 RR-16460/RR/NCRR NIH HHS/United States
- P30 NS047546/NS/NINDS NIH HHS/United States
- P20 RR016460/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous