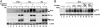Extensive phosphorylation of Smoothened in Hedgehog pathway activation - PubMed (original) (raw)
Extensive phosphorylation of Smoothened in Hedgehog pathway activation
Chi Zhang et al. Proc Natl Acad Sci U S A. 2004.
Abstract
The transmembrane protein Smoothened (Smo) is activated in response to the extracellular protein signal, Hedgehog (Hh), and transmits this state of pathway activity into the cell. Previous studies in Drosophila have correlated pathway activation with Smo accumulation and increased phosphorylation. Using immunopurification and mass spectrometry, we identify here 26 serine/threonine residues within the Smo C-terminal cytoplasmic tail that are phosphorylated in Hh-stimulated cells. By systematically substituting alanine or glutamic acid to block or simulate phosphorylation, we provide evidence for a functional role of collective phosphorylation of a subset of phosphoresidues in pathway activation. This role is indicated by the ability of altered Smo proteins to produce changes in transcription of Hh-responsive genes in vivo and in cultured cells. These altered Smo proteins also affect biochemical indicators of pathway activity, such as Smo accumulation and phosphorylation of other pathway components. The prevalence and arrangement of phosphoresidues within the Smo cytoplasmic tail at recognition sites for cAMP-dependent protein kinase and casein kinase 1 suggest a role for these kinases in Smo phosphorylation, and such a role is supported by the effects of manipulating kinase activities in cultured cells. Our studies confirm and extend previous studies showing a positive effect for cAMP-dependent protein kinase and uncover a positive role for casein kinase 1alpha in Hh pathway activation.
Figures
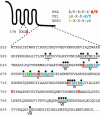
Fig. 1.
Predicted and detected phosphorylation in Smo. A schematic diagram of Smo is shown, alongside specific residues within intracellular loop two and a portion of the C-terminal cytoplasmic tail. Identified phosphoserine/threonine residues in endogenous Smo purified from HhN-stimulated Drosophila S2 cells are marked with asterisks. Phosphopeptides were isolated from eight distinct regions, as designated (I–VIII). Residues within the consensus kinase recognition motifs shown for PKA (red) and CK1 (blue) are highlighted. One residue that may be phosphorylated by either CK1 or GSK3, depending on the priming phosphoresidue, is shown in green. Designations of CK1 and GSK3 target residues assume that all possible priming phosphorylation has occurred.
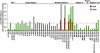
Fig. 2.
Mutation of Smo phosphorylation sites modulates Hh-responsive reporter expression. Hh pathway responsiveness was monitored by using the ptc-Luc reporter after transfection with dsRNA targeting the smo 5′ UTR to deplete endogenous smo transcript and plasmids to express mutant smo alleles. See Table 2 for details on the smo alleles shown. Shown is the normalized reporter activity produced by each variant relative to that of wild-type Smo in the presence of HhN (“+HhN”) (set to a value of 1; dotted line). Each variant was tested in a minimum of two independent assays, and the results were averaged.
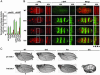
Fig. 3.
Smo phosphorylation site variants show transdominant effects in vitro and in vivo. (A) Overexpressed untagged and GFP-tagged Smo variants cause transdominant effects on reporter activity. Either pAcSV- or pUAST-based plasmids expressing the Smo variants shown were transfected in the presence of endogenous Smo. The results of a representative ptc-Luc reporter assay are shown. (B) Overexpression of GFP-tagged Smo-Glu in embryos leads to expansion of Wg expression. Immunofluorescence using anti-GFP (green) and anti-Wg (red) Abs is shown for dorsal views at ×10 and ×25 of embryos at extended germ-band stage that are either wild-type (WT; w1118) or expressing UAS GFP-tagged wild-type (Smo), III,V,VI Ala (Ala), or III,V,VI Glu (Glu) forms of Smo using the prd-GAL4 driver. P ↔ A, orientation of the posterior/anterior axis. (C) Overexpression of Smo variants leads to wing patterning alterations. Wings collected from adult flies that are either wild-type (WT) or heterozygous for the ptc-GAL4 (Upper) or 71B GAL4 (Lower) driver and expressing GFP-Smo variants as in B are shown. Longitudinal veins 1–5 are labeled. Asterisk, proximal L3 and L4 fusion for the GFP-Smo-Ala variant overexpressed with ptc-GAL4; arrowheads, ectopic veination near the proximal end of L3 when GFP-Smo is overexpressed (black) and proximal L3 and L4 fusion when GFP-Smo-Ala is overexpressed (white) using 71B GAL4. Flies expressing GFP-Smo-Glu die as pupae with _ptc-_GAL4 and as pharate adults with 71B GAL4. A severely defective wing from an escaper from the GFP-Smo-Glu 71B GAL4 line is shown (Lower Right).
Fig. 4.
Phosphorylation in modulation of Smo stability and signaling activity. (A) Smo variants show different levels of Smo accumulation and phosphorylation of pathway components. S2R+ cells were transfected with expression constructs for wild-type Myc-Smo (Smo); Myc-Smo-III,V,VI Ala (Ala); Myc-Smo-III,V,VI Glu (Glu); Myc-Cos2; MycHis-Fu; V5-Su(fu); and/or pAct5C GAL4/UAS-hh (Hh). Approximately 48 h after transfection, lysates were analyzed by Western blotting with anti-Myc, anti-V5, or anti-lamin Ab. The lamin signal serves as a loading control. Arrowheads, dephosphorylated or minimally phosphorylated forms; bars, heavily phosphorylated species. (B) Levels of PKA and CK1α activity influence accumulation and phosphorylation of Smo. S2R+ cells were transfected with expression constructs for GFP (-); wild-type Myc-Smo (Smo); a constitutively active mouse PKA catalytic subunit (mC*); and/or wild-type or kinase-inactive (K/R) CK1α, as shown. Cells were treated with control (-) or HhN conditioned (+) medium for ∼24 h before lysis and analysis of lysates by Western blotting with anti-Myc or anti-tubulin Ab. Tubulin signal serves as a loading control. Arrowhead, minimally phosphorylated Smo; bar, heavily phosphorylated Smo.
References
- Ingham, P. W. & McMahon, A. P. (2001) Genes Dev. 15, 3059–3087. - PubMed
- Lum, L. & Beachy, P. A. (2004) Science 304, 1755–1759. - PubMed
- Beachy, P. A., Karhadkar, S. S. & Berman, D. M. (2004) Nature 432, 324–331. - PubMed
- Marigo, V., Davey, R. A., Zuo, Y., Cunningham, J. M. & Tabin, C. J. (1996) Nature 384, 176–179. - PubMed
- Stone, D. M., Hynes, M., Armanini, M., Swanson, T. A., Gu, Q., Johnson, R. L., Scott, M. P., Pennica, D., Goddard, A., Phillips, H., et al. (1996) Nature 384, 129–134. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous