Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88 - PubMed (original) (raw)
Fatal Mycobacterium tuberculosis infection despite adaptive immune response in the absence of MyD88
Cecile M Fremond et al. J Clin Invest. 2004 Dec.
Abstract
Toll-like receptors (TLRs) such as TLR2 and TLR4 have been implicated in host response to mycobacterial infection. Here, mice deficient in the TLR adaptor molecule myeloid differentiation factor 88 (MyD88) were infected with Mycobacterium tuberculosis (MTB). While primary MyD88(-/-) macrophages and DCs are defective in TNF, IL-12, and NO production in response to mycobacterial stimulation, the upregulation of costimulatory molecules CD40 and CD86 is unaffected. Aerogenic infection of MyD88(-/-) mice with MTB is lethal within 4 weeks with 2 log(10) higher CFU in the lung; high pulmonary levels of cytokines and chemokines; and acute, necrotic pneumonia, despite a normal T cell response with IFN-gamma production to mycobacterial antigens upon ex vivo restimulation. Vaccination with Mycobacterium bovis bacillus Calmette-Guerin conferred a substantial protection in MyD88(-/-) mice from acute MTB infection. These data demonstrate that MyD88 signaling is dispensable to raise an acquired immune response to MTB. Nonetheless, this acquired immune response is not sufficient to compensate for the profound innate immune defect and the inability of MyD88(-/-) mice to control MTB infection.
Figures
Figure 1
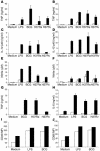
Impaired proinflammatory cytokine and NO production in MyD88–/– macrophages and DCs. BM-derived macrophages (A, C, and E) and DCs (B, D, and F) (5 × 105 cells/ml) prepared from MyD88–/– (white bars) and wild-type (black bars) mice were incubated with LPS (100 ng/ml), M. bovis BCG, MTB H37Ra, or MTB H37Rv (all at 2 bacteria per cell). After 24 hours, the production of TNF (A and B), IL-12 p40 (C and D), or nitrite (E and F) was determined in the supernatant by ELISA or Griess reaction. TNF and IL-6 production by pulmonary macrophages stimulated in the same conditions was also measured (G and H). Upregulation of CD40 (I) and CD86 (J) expression by DCs stimulated with LPS or M. bovis BCG were analyzed by FACS. Data are from 1 experiment, representative of 3 independent experiments with n = 2 mice per genotype; mean values ± SD are shown. MFI, mean fluorescence intensity.
Figure 2
MyD88–/– mice are unable to control an MTB infection. MyD88–/– (open circles), TNF–/– (open squares), and wild-type (filled squares) mice were exposed to a low aerogenic dose of MTB H37Rv (200 CFU per mouse i.n.) and monitored for body weight (A; mean values of n = 6–8 mice per group from 1 representative experiment out of 7 independent experiments) and survival (B; n = 13–24 mice pooled from several experiments; P – 0.01 between MyD88–/– or TNF–/– mice and wild-type controls, and between TNF- and MyD88-deficient mice). (C) Lung wet weight of TNF–/– mice (gray bars), MyD88–/– mice (white bars), and wild-type controls (black bars) were measured 17 and 27 days after infection. The numbers of viable bacteria present in the lungs (D) and spleen (E) of MyD88–/– (white bars) and wild-type (black bars) mice were determined after 27 days of infection (mean ± SD of n = 3–4 lung or n = 5–7 spleen samples from 1 representative experiment out of 2 or 3 independent experiments, respectively; **P – 0.01).
Figure 3
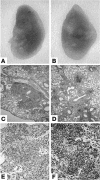
MyD88–/– mice exhibit acute necrotic pneumonia with large nodules but defective granuloma formation in response to MTB infection. Lung tissue from MyD88–/– (A, C, E, and G) and wild-type (B, D, F, and H) mice was analyzed 27 days after MTB H37Rv infection (200 CFU i.n.). Lungs of MyD88–/– mice showed large and confluent nodules (A) in comparison with those of wild-type mice (B). Microscopic examination showed extensive inflammation and necrosis in infected MyD88–/– lungs (C–F; H&E) with abundant mycobacteria in the extracellular space (G and H; Ziehl-Neelsen). Low-power micrographs of representative lung sections are shown in C and D (magnification, ×50), and higher magnification shows details in E–H (magnification, ×400).
Figure 4
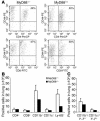
T lymphocyte and myeloid cell recruitment and activation in the lung of MyD88–/– infected mice. Infiltrating cells from the lungs of MyD88–/– and control mice were isolated 27 days after infection and analyzed by flow cytometry for the expression of CD4, CD8, CD44, CD11b, CD11c, Ly-6G, and MHC class II IA–IE. Typical dot plots of FACS analysis are shown for CD44 expression in CD4+ and CD8+ T cells (A; gated on T lymphocytes). Graphic representations of the different leukocyte populations (B) and of the expression of MHC class II on APCs (C) are shown. Results are expressed as absolute numbers of positive cells. Mean ± SD from 2 MyD88–/– mice are shown and are representative of 2 independent experiments.
Figure 5
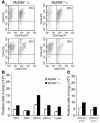
Reduced DTH response, but normal IFN-γ secretion, to mycobacterial antigens stimulation in MyD88–/– mice. (A) Cutaneous DTH response was performed 3 weeks after vaccination with M. bovis BCG (105 CFU s.c.; v) by assessing the footpad swelling in response to PPD injection as described. Paw swelling is defined as the difference in thickness between the left paw injected with PPD and the right paw injected with saline. DTH responses of nonvaccinated MyD88–/– and MyD88+/+ mice are shown as control (nv). Data are expressed as mean ± SD (n = 7–8) and are from 1 representative experiment out of 2. **P – 0.01. (B) For the T cell response, spleen cells from M. bovis BCG–vaccinated MyD88-deficient and control mice were harvested 4 weeks after inoculation (105 CFU s.c.) and were restimulated in vitro in the presence of soluble BCG antigens (SupBCG, 10 μg/ml) or unrelated antigen (HKLM, 100 bacteria per cell). Naive MyD88–/– mice and wild-type mice were used as negative control. IFN-γ production was quantified in the supernatants after 48 hours of incubation. Results are mean ± SD from n = 2 mice per genotype and are representative of 3 independent experiments. (C) Intracellular IFN-γ staining of CD4+ or CD8+ splenocytes from BCG-infected MyD88-deficient and control mice 4 weeks after infection, restimulated for 18 hours in the presence of soluble BCG antigens (SupBCG, 10 μg/ml) or unrelated antigen (HKLM, 100 bacteria per cell). Typical dot blots are shown for CD4+ T cells (numbers indicate percentage of IFN-γ–positive CD4+ cells).
Figure 6
BCG vaccination confers an initial protection to MTB-challenged MyD88–/– mice. MyD88–/– mice and control mice were either immunized by s.c. injection of M. bovis BCG (105 CFU) or left naive and 5 weeks later challenged by MTB H37Rv aerogenic infection (200 CFU per mouse i.n.). The mice were monitored for body weight (A) or survival (B) or were sacrificed at 4 weeks after MTB challenge and analyzed for lung weight (C) and bacterial counts (D) as in Figure 2 (A: n = 7–8 mice per group from 1 representative experiment out of 2; B: n = 11–13 mice per group from 2 experiments; C and D: n = 4 per group from 1 representative experiment out of 3; *P – 0.05; **P – 0.01).
Figure 7
BCG vaccination prevents necrotic pneumonia by aerogenic MTB in MyD88–/– mice. Lung tissue from BCG-vaccinated MyD88–/– (A, C, and E) and wild-type (B, D, and F) mice was analyzed at 4 weeks after MTB H37Rv infection (200 CFU i.n.). Lungs of MyD88–/– mice display small nodules (A), similar to vaccinated wild-type mice (B). Microscopic examination reveals a striking increase of mononuclear cells including abundant lymphocytes in the lungs of vaccinated and infected MyD88–/– and wild-type mice (C–F; H&E). Low-power micrographs of representative lung sections are shown in C and D (magnification, ×50), and higher magnification shows details in E and F (magnification, ×400).
Figure 8
Cell recruitment in the lungs of vaccinated and MTB-challenged MyD88–/– mice. MyD88–/– and control mice were immunized by s.c. injection of M. bovis BCG (105 CFU) and 5 weeks later challenged by MTB aerogenic infection (200 CFU) as in Figure 6. Leukocytes from infected lung were isolated 4 weeks after infection and analyzed by flow cytometry for CD44 expression in CD4- and CD8-positive T cells (A and B); for CD11b-, CD11c-, and Ly-6G–positive cells (B); and for MHC class II IA–IE expression (C) as in Figure 4. Results are expressed as absolute numbers of positive cells. Mean ± SD from 2 MyD88–/– mice are shown and are representative of 2 independent experiments.
Figure 9
The high pulmonary levels of cytokines and chemokines in MTB-infected MyD88–/– mice is reduced upon vaccination. Cytokine and chemokine concentrations were determined in lung homogenates from MyD88–/– and control mice immunized by s.c. injection of M. bovis BCG (105 CFU) and challenged 5 weeks later by MTB aerogenic infection (200 CFU) as in Figure 6. IL-1β (A), IFN-γ (B), TNF (C), MIP-1α (D), MCP-1 (E), and RANTES (F) were quantified by SearchLight protein array. Results are expressed as mean ± SD from 4 mice per group (*P – 0.05; **P – 0.01).
Comment in
- TB, or not TB: that is the question -- does TLR signaling hold the answer?
Doherty TM, Arditi M. Doherty TM, et al. J Clin Invest. 2004 Dec;114(12):1699-703. doi: 10.1172/JCI23867. J Clin Invest. 2004. PMID: 15599394 Free PMC article. Review.
Similar articles
- Chronic pneumonia despite adaptive immune response to Mycobacterium bovis BCG in MyD88-deficient mice.
Nicolle DM, Pichon X, Bouchot A, Maillet I, Erard F, Akira S, Ryffel B, Quesniaux VF. Nicolle DM, et al. Lab Invest. 2004 Oct;84(10):1305-21. doi: 10.1038/labinvest.3700149. Lab Invest. 2004. PMID: 15258598 - IL-1 receptor-mediated signal is an essential component of MyD88-dependent innate response to Mycobacterium tuberculosis infection.
Fremond CM, Togbe D, Doz E, Rose S, Vasseur V, Maillet I, Jacobs M, Ryffel B, Quesniaux VF. Fremond CM, et al. J Immunol. 2007 Jul 15;179(2):1178-89. doi: 10.4049/jimmunol.179.2.1178. J Immunol. 2007. PMID: 17617611 - MyDths and un-TOLLed truths: sensor, instructive and effector immunity to tuberculosis.
Reiling N, Ehlers S, Hölscher C. Reiling N, et al. Immunol Lett. 2008 Feb 15;116(1):15-23. doi: 10.1016/j.imlet.2007.11.015. Epub 2007 Dec 26. Immunol Lett. 2008. PMID: 18191460 Review. - TB, or not TB: that is the question -- does TLR signaling hold the answer?
Doherty TM, Arditi M. Doherty TM, et al. J Clin Invest. 2004 Dec;114(12):1699-703. doi: 10.1172/JCI23867. J Clin Invest. 2004. PMID: 15599394 Free PMC article. Review.
Cited by
- MyD88-dependent signaling influences fibrosis and alternative macrophage activation during Staphylococcus aureus biofilm infection.
Hanke ML, Angle A, Kielian T. Hanke ML, et al. PLoS One. 2012;7(8):e42476. doi: 10.1371/journal.pone.0042476. Epub 2012 Aug 3. PLoS One. 2012. PMID: 22879997 Free PMC article. - The role of airway epithelial cells in response to mycobacteria infection.
Li Y, Wang Y, Liu X. Li Y, et al. Clin Dev Immunol. 2012;2012:791392. doi: 10.1155/2012/791392. Epub 2012 Apr 18. Clin Dev Immunol. 2012. PMID: 22570668 Free PMC article. Review. - CD14 contributes to pulmonary inflammation and mortality during murine tuberculosis.
Wieland CW, van der Windt GJ, Wiersinga WJ, Florquin S, van der Poll T. Wieland CW, et al. Immunology. 2008 Oct;125(2):272-9. doi: 10.1111/j.1365-2567.2008.02840.x. Epub 2008 Apr 3. Immunology. 2008. PMID: 18393969 Free PMC article. - Immune subversion by Mycobacterium tuberculosis through CCR5 mediated signaling: involvement of IL-10.
Das S, Banerjee S, Majumder S, Chowdhury BP, Goswami A, Halder K, Chakraborty U, Pal NK, Majumdar S. Das S, et al. PLoS One. 2014 Apr 2;9(4):e92477. doi: 10.1371/journal.pone.0092477. eCollection 2014. PLoS One. 2014. PMID: 24695099 Free PMC article. - Transient Receptor Potential Melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes.
Knowles H, Heizer JW, Li Y, Chapman K, Ogden CA, Andreasen K, Shapland E, Kucera G, Mogan J, Humann J, Lenz LL, Morrison AD, Perraud AL. Knowles H, et al. Proc Natl Acad Sci U S A. 2011 Jul 12;108(28):11578-83. doi: 10.1073/pnas.1010678108. Epub 2011 Jun 27. Proc Natl Acad Sci U S A. 2011. PMID: 21709234 Free PMC article.
References
- Dye C, Williams BG, Espinal MA, Raviglione MC. Erasing the world’s slow stain: strategies to beat multidrug-resistant tuberculosis. Science. 2002;295:2042–2046. - PubMed
- Flynn JL, Chan J. Immunology of tuberculosis. Annu. Rev. Immunol. 2001;19:93–129. - PubMed
- Akira S. Mammalian Toll-like receptors. Curr. Opin. Immunol. 2003;15:5–11. - PubMed
- Aliprantis AO, et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. - PubMed
- Thoma-Uszynski S, et al. Induction of direct antimicrobial activity through mammalian toll-like receptors. Science. 2001;291:1544–1547. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials