tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors - PubMed (original) (raw)
. 2005 Jan 11;102(2):407-12.
doi: 10.1073/pnas.0406252102. Epub 2005 Jan 3.
Ryan D Murphey, Erno Wienholds, Donna Neuberg, Jeffery L Kutok, Christopher D M Fletcher, John P Morris, Ting Xi Liu, Stefan Schulte-Merker, John P Kanki, Ronald Plasterk, Leonard I Zon, A Thomas Look
Affiliations
- PMID: 15630097
- PMCID: PMC544293
- DOI: 10.1073/pnas.0406252102
tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors
Stéphane Berghmans et al. Proc Natl Acad Sci U S A. 2005.
Abstract
TP53 is the most frequently mutated tumor suppressor gene in human cancer, with nearly 50% of all tumors exhibiting a loss-of-function mutation. To further elucidate the genetic pathways involving TP53 and cancer, we have exploited the zebrafish, a powerful vertebrate model system that is amenable to whole-genome forward-genetic analysis and synthetic-lethal screens. Zebrafish lines harboring missense mutations in the tp53 DNA-binding domain were identified by using a target-selected mutagenesis strategy. Homozygous mutant fish from two of these lines were viable and exhibited mutations similar to those found in human cancers (tp53(N168K) and tp53(M214K)). Although homozygous tp53(N168K) mutants were temperature-sensitive and suppressed radiation-induced apoptosis only at 37 degrees C, cells in the tp53(M214K) embryos failed to undergo apoptosis in response to gamma radiation at both 28 and 37 degrees C. Unlike wild-type control embryos, irradiated tp53(M214K) embryos also failed to up-regulate p21 and did not arrest at the G(1)/S checkpoint. Beginning at 8.5 months of age, 28% of tp53(M214K) mutant fish developed malignant peripheral nerve sheath tumors. In addition to providing a model for studying the molecular pathogenic pathways of malignant peripheral nerve sheath tumors, these mutant zebrafish lines provide a unique platform for modifier screens to identify genetic mutations or small molecules that affect tp53-related pathways, including apoptosis, cell-cycle delay, and tumor suppression.
Figures
Fig. 1.
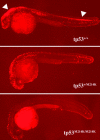
Induction of apoptosis by γ irradiation is suppressed in tp53 mutant embryos. Embryos were γ irradiated at 24 hpf (16 Gray) and fixed at 30 hpf for TUNEL assay. After the assay of multiple embryos resulting from the intercross of _tp53_M214K mutant heterozygous parents, the embryos were genotyped for their tp53 status. Embryos shown are representative of homozygous wild-type (tp53+/+), heterozygous (tp53+/M214K), and homozygous mutant (_tp53_M214K/M214K) genotypes. White arrows indicate widespread apoptosis in the brain and spinal cord. Embryos were raised at 28°C. All embryos are shown in lateral view with the head to the left.
Fig. 2.
_tp53_M214K embryos have defects in the γ irradiation-induced G1 checkpoint. DNA content analysis was performed on homozygous _tp53_M214K mutant (Lower) and wild-type sibling (Upper) embryos at 0, 3, 5, 7, and 24 h postirradiation (16 Gray). Results are representative of at least three experiments.
Fig. 3.
tp53 mutant embryos lack up-regulation of key downstream target genes after γ irradiation. Genes involved in the tp53 regulatory (tp53, mdm2), cell-cycle checkpoint (p21), and apoptotic (bax) pathways were analyzed in homozygous, heterozygous, and wild-type embryos with (+) or without (-) γ irradiation at 24 hpf (16 Gray). Gene expression was assayed at 30 hpf by single-embryo RT-PCR. Results are representative of at least five experiments. β actin was used to control gene expression.
Fig. 4.
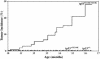
_tp53_M214K mutant zebrafish develop zMPNST at high frequency. Tumor incidence curves show cohorts of homozygous mutant (tp53M214K/M214K), heterozygous (tp53M214K/+), and wild type (tp53+/+).
Fig. 5.
Tumorigenesis features of _tp53_M214K mutant zebrafish. (A-D) When compared with wild-type zebrafish (A and C), zMPNST development in tp53 mutant zebrafish is identifiable upon external observation because of ocular (B) or abdominal tumor localizations (D). (E-H) When compared with wild-type zebrafish (E and G), histopathology staining with hematoxylin/eosin reveals zMPNST in the eye (F) and abdominal cavity (H), as indicated by the stars (×4). (I-K) Histopathological features of tumors (I) composed predominantly of spindle cells (J) and to a varying degree of epitheloid cells (K) are consistent with the diagnosis of zMPNST. [Bar, 200 μm (E, F, I-K).]
Similar articles
- BRCA2 and TP53 collaborate in tumorigenesis in zebrafish.
Shive HR, West RR, Embree LJ, Golden CD, Hickstein DD. Shive HR, et al. PLoS One. 2014 Jan 29;9(1):e87177. doi: 10.1371/journal.pone.0087177. eCollection 2014. PLoS One. 2014. PMID: 24489863 Free PMC article. - Histologic and Immunohistochemical Analyses of Soft Tissue Sarcomas From brca2-Mutant/ tp53-Mutant Zebrafish Are Consistent With Neural Crest (Schwann Cell) Origin.
White LA, Sexton JM, Shive HR. White LA, et al. Vet Pathol. 2017 Mar;54(2):320-327. doi: 10.1177/0300985816669406. Epub 2016 Nov 24. Vet Pathol. 2017. PMID: 27879444 - Inferior survival for patients with malignant peripheral nerve sheath tumors defined by aberrant TP53.
Høland M, Kolberg M, Danielsen SA, Bjerkehagen B, Eilertsen IA, Hektoen M, Mandahl N, van den Berg E, Smeland S, Mertens F, Sundby Hall K, Picci P, Sveen A, Lothe RA. Høland M, et al. Mod Pathol. 2018 Nov;31(11):1694-1707. doi: 10.1038/s41379-018-0074-y. Epub 2018 Jun 26. Mod Pathol. 2018. PMID: 29946184 - [TP53 mutations and molecular epidemiology].
Otsuka K, Ishioka C. Otsuka K, et al. Gan To Kagaku Ryoho. 2007 May;34(5):683-9. Gan To Kagaku Ryoho. 2007. PMID: 17496437 Review. Japanese. - TP53 tumour suppressor gene: clues to molecular carcinogenesis and cancer therapy.
Wang XW, Harris CC. Wang XW, et al. Cancer Surv. 1996;28:169-96. Cancer Surv. 1996. PMID: 8977035 Review.
Cited by
- Gpr125 modulates Dishevelled distribution and planar cell polarity signaling.
Li X, Roszko I, Sepich DS, Ni M, Hamm HE, Marlow FL, Solnica-Krezel L. Li X, et al. Development. 2013 Jul;140(14):3028-39. doi: 10.1242/dev.094839. Development. 2013. PMID: 23821037 Free PMC article. - Zebrafish Tumor Graft Transplantation to Grow Tumors In Vivo That Engraft Poorly as Single Cell Suspensions.
Lipsitt A, Hensch NR, Moreno-Campos R, Baxi K, Chen J, Challa AK, Chen EY, Ignatius MS. Lipsitt A, et al. Zebrafish. 2021 Aug;18(4):293-296. doi: 10.1089/zeb.2021.0006. Epub 2021 May 24. Zebrafish. 2021. PMID: 34030492 Free PMC article. - Autophagy induction is a Tor- and Tp53-independent cell survival response in a zebrafish model of disrupted ribosome biogenesis.
Boglev Y, Badrock AP, Trotter AJ, Du Q, Richardson EJ, Parslow AC, Markmiller SJ, Hall NE, de Jong-Curtain TA, Ng AY, Verkade H, Ober EA, Field HA, Shin D, Shin CH, Hannan KM, Hannan RD, Pearson RB, Kim SH, Ess KC, Lieschke GJ, Stainier DY, Heath JK. Boglev Y, et al. PLoS Genet. 2013;9(2):e1003279. doi: 10.1371/journal.pgen.1003279. Epub 2013 Feb 7. PLoS Genet. 2013. PMID: 23408911 Free PMC article. - p53 isoform Δ113p53 promotes zebrafish heart regeneration by maintaining redox homeostasis.
Ye S, Zhao T, Zhang W, Tang Z, Gao C, Ma Z, Xiong JW, Peng J, Tan WQ, Chen J. Ye S, et al. Cell Death Dis. 2020 Jul 23;11(7):568. doi: 10.1038/s41419-020-02781-7. Cell Death Dis. 2020. PMID: 32703938 Free PMC article. - Mutations in FAM50A suggest that Armfield XLID syndrome is a spliceosomopathy.
Lee YR, Khan K, Armfield-Uhas K, Srikanth S, Thompson NA, Pardo M, Yu L, Norris JW, Peng Y, Gripp KW, Aleck KA, Li C, Spence E, Choi TI, Kwon SJ, Park HM, Yu D, Heo WD, Mooney MR, Baig SM, Wentzensen IM, Telegrafi A, McWalter K, Moreland T, Roadhouse C, Ramsey K, Lyons MJ, Skinner C, Alexov E, Katsanis N, Stevenson RE, Choudhary JS, Adams DJ, Kim CH, Davis EE, Schwartz CE. Lee YR, et al. Nat Commun. 2020 Jul 23;11(1):3698. doi: 10.1038/s41467-020-17452-6. Nat Commun. 2020. PMID: 32703943 Free PMC article.
References
- Levine, A. J. (1997) Cell 88, 323-331. - PubMed
- Vousden, K. H. (2000) Cell 103, 691-694. - PubMed
- Vogelstein, B., Lane, D. & Levine, A. J. (2000) Nature 408, 307-310. - PubMed
- Kastan, M. B., Onyekwere, O., Sidransky, D., Vogelstein, B. & Craig, R. W. (1991) Cancer Res. 51, 6304-6311. - PubMed
- Vousden, K. H. & Lu, X. (2002) Nat. Rev. Cancer 2, 594-604. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous