Glucosamine induces cell-cycle arrest and hypertrophy of mesangial cells: implication of gangliosides - PubMed (original) (raw)
Glucosamine induces cell-cycle arrest and hypertrophy of mesangial cells: implication of gangliosides
Elodie Masson et al. Biochem J. 2005.
Abstract
Alterations in proliferation and hypertrophy of renal mesangial cells are typical features of diabetic nephropathy. The HP (hexosamine pathway) has been proposed as a biochemical hypothesis to explain microvascular alterations due to diabetic nephropathy; however, involvement of HP in the regulation of mesangial cell growth or hypertrophy has been poorly studied. Although gangliosides are known to regulate cell proliferation, their potential role in mesangial cell-growth perturbations has hardly been explored. In the present study, we investigated the effects of the HP activation, mimicked by GlcN (glucosamine) treatment, on mesangial cell growth and hypertrophy and the potential implication of gangliosides in these processes. Our results indicate that GlcN induced hypertrophy of mesangial cells, as measured by an increase in the protein/cell ratio, and it caused cell-cycle arrest by an increase in the expression of cyclin-dependent kinase inhibitor p21(Waf1/Cip1). Furthermore, GlcN treatment resulted in a massive increase in the levels of gangliosides G(M2) and G(M1). Treatment of cells with exogenous G(M2) and G(M1) reproduced the effects of 0.5 mM GlcN on p21(Waf1/Cip1) expression, cell-cycle arrest and hypertrophy, suggesting that gangliosides G(M2) and G(M1) are probably involved in mediating GlcN effects. These results document a new role of the HP in the regulation of mesangial cell growth and hypertrophy. They also suggest a potential new mechanism of action of the HP through modulation of ganglioside levels.
Figures
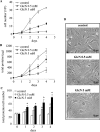
Figure 1. GlcN decreases RMC growth and induces hypertrophy
Mesangial cells were exposed to 0.5 or 5 mM GlcN for 4 days. On each day, the cells were collected, cell number was counted and total protein content was measured. Growth curves corresponding to cell number (expressed in 105 cells) (A) and total protein content (expressed in μg) (B) are shown. The total protein/cell number ratio is presented in (C), expressed in μg of protein/105 cells. Results are the means±S.E.M. for four independent experiments. (D) Microscopy phase images of cells under control conditions or cells treated with GlcN. *P<0.05 versus control.
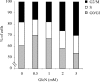
Figure 2. Dose-dependent effects of GlcN on cell-cycle arrest
RMC were treated with increasing concentrations of GlcN for 48 h. Cells were then collected and analysed by flow cytometry as described in the Materials and methods section. Results are expressed as percentage of cells in G0/G1, S and G2/M phases.
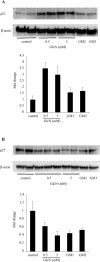
Figure 3. GlcN, GM2 and GM1 increase p21Waf1/Cip1 expression
RMC were exposed to GlcN (0.5 and 5 mM), GM2 (60 μM) and GM1 (60 μM) for 48 h. Cells were then lysed and equal amounts of protein were immunoblotted with monoclonal anti-p21Waf1/Cip1 (A) or anti-p27Kip1 (B) antibodies. Membranes were immunoblotted with anti-β-actin to control for equal protein loading and transfer. Representative immunoblots and quantification obtained by densitometric analysis of the bands are shown. Results were corrected to β-actin signal and are expressed as fold change compared with control. Results are the means±S.E.M. for four independent experiments.
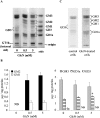
Figure 4. Effect of GlcN on ganglioside profile
(A, B) RMC were treated with GlcN (0.5 and 5 mM) for 48 h and then collected. Gangliosides were extracted, purified and then analysed by HPTLC and resorcinol staining. Representative HPTLC (A) and densitometric quantification (B) are shown. Results are expressed as μg of gangliosides/mg of protein and are the means±S.E.M. for four independent experiments each performed in duplicate. Under control conditions, ganglioside quantities were 4.62±0.37, 1.65±0.11 and 0.30±0.05 μg/mg of protein for GM3, GD1a and GD3 respectively. ND, not detectable. *P<0.05 versus control. (C) RMC were labelled for 48 h in the presence of 0.2 μCi/ml [14C]GlcN for control cells and 2 mM GlcN at a specific activity of 0.5 mCi/ml for treated cells. At the end of incubation, cells were washed extensively and gangliosides were extracted, purified and separated by HPTLC as described in the Experimental section. Radioactivity associated with gangliosides was then analysed by autoradiography of the HPTLC plates.
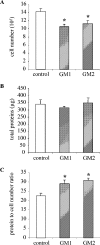
Figure 5. GM2 and GM1 gangliosides induce mesangial cell hypertrophy
RMC were cultured in the presence of GM2 or GM1 (60 μM) for 48 h. Control conditions were complete medium with a mixture of BSA and 10 mM Hepes (pH 7.4). Cells were then harvested and counted using a haemocytometer and total protein content was measured. Cell number (expressed for 105 cells) (A) and total protein content (expressed in μg) (B) are presented. The total protein/cell number ratio used to assess hypertrophy is expressed in μg of protein/105 cells (C). Results are the means±S.E.M. for 4–6 independent experiments. *P<0.05 versus control.
Similar articles
- Hexosamine induction of oxidative stress, hypertrophy and laminin expression in renal mesangial cells: effect of the anti-oxidant alpha-lipoic acid.
Singh LP, Cheng DW, Kowluru R, Levi E, Jiang Y. Singh LP, et al. Cell Biochem Funct. 2007 Sep-Oct;25(5):537-50. doi: 10.1002/cbf.1358. Cell Biochem Funct. 2007. PMID: 16892452 - Involvement of gangliosides in glucosamine-induced proliferation decrease of retinal pericytes.
Masson E, Wiernsperger N, Lagarde M, El Bawab S. Masson E, et al. Glycobiology. 2005 Jun;15(6):585-91. doi: 10.1093/glycob/cwi039. Epub 2004 Dec 29. Glycobiology. 2005. PMID: 15625180 - Hyperglycemia and glucosamine-induced mesangial cell cycle arrest and hypertrophy: Common or independent mechanisms?
Masson E, Lagarde M, Wiernsperger N, El Bawab S. Masson E, et al. IUBMB Life. 2006 Jul;58(7):381-8. doi: 10.1080/15216540600755980. IUBMB Life. 2006. PMID: 16801212 Review. - Role of growth arrest-specific gene 6 in diabetic nephropathy.
Arai H, Nagai K, Doi T. Arai H, et al. Vitam Horm. 2008;78:375-92. doi: 10.1016/S0083-6729(07)00015-5. Vitam Horm. 2008. PMID: 18374201 Review.
Cited by
- Alpha-lipoic acid upregulates antioxidant enzyme gene expression and enzymatic activity in diabetic rat kidneys through an O-GlcNAc-dependent mechanism.
Arambašić J, Mihailović M, Uskoković A, Dinić S, Grdović N, Marković J, Poznanović G, Bajec D, Vidaković M. Arambašić J, et al. Eur J Nutr. 2013 Aug;52(5):1461-73. doi: 10.1007/s00394-012-0452-z. Epub 2012 Oct 12. Eur J Nutr. 2013. PMID: 23064900 - Glucosamine suppresses proliferation of human prostate carcinoma DU145 cells through inhibition of STAT3 signaling.
Chesnokov V, Sun C, Itakura K. Chesnokov V, et al. Cancer Cell Int. 2009 Sep 10;9:25. doi: 10.1186/1475-2867-9-25. Cancer Cell Int. 2009. PMID: 19744341 Free PMC article. - The sweet side of the cell cycle.
Tan EP, Duncan FE, Slawson C. Tan EP, et al. Biochem Soc Trans. 2017 Apr 15;45(2):313-322. doi: 10.1042/BST20160145. Biochem Soc Trans. 2017. PMID: 28408472 Free PMC article. Review. - ER Stress-Perturbed Intracellular Protein O-GlcNAcylation Aggravates Podocyte Injury in Diabetes Nephropathy.
Song S, Hu T, Shi X, Jin Y, Liu S, Li X, Zou W, Wang C. Song S, et al. Int J Mol Sci. 2023 Dec 18;24(24):17603. doi: 10.3390/ijms242417603. Int J Mol Sci. 2023. PMID: 38139429 Free PMC article. - Hyperglycemia-Induced Aberrant Cell Proliferation; A Metabolic Challenge Mediated by Protein O-GlcNAc Modification.
Nagy T, Fisi V, Frank D, Kátai E, Nagy Z, Miseta A. Nagy T, et al. Cells. 2019 Aug 28;8(9):999. doi: 10.3390/cells8090999. Cells. 2019. PMID: 31466420 Free PMC article. Review.
References
- Trevisan R., Barnes D. J., Viberti G. Pathogenesis of diabetic nephropathy. In: Pickup J., Williams G., editors. Textbook of Diabetes. Oxford, U.K.: Blackwell Science; 1997. pp. 52.1–52.21.
- Wolf G. Cell cycle regulation in diabetic nephropathy. Kidney Int. Suppl. 2000;77:S59–S66. - PubMed
- Kuan C. J., al Douahji M., Shankland S. J. The cyclin kinase inhibitor p21WAF1, CIP1 is increased in experimental diabetic nephropathy: potential role in glomerular hypertrophy. J. Am. Soc. Nephrol. 1998;9:986–993. - PubMed
- Wolf G., Schroeder R., Ziyadeh F. N., Thaiss F., Zahner G., Stahl R. A. High glucose stimulates expression of p27Kip1 in cultured mouse mesangial cells: relationship to hypertrophy. Am. J. Physiol. 1997;273:F348–F356. - PubMed
- Wolf G., Schroeder R., Thaiss F., Ziyadeh F. N., Helmchen U., Stahl R. A. Glomerular expression of p27Kip1 in diabetic db/db mouse: role of hyperglycemia. Kidney Int. 1998;53:869–879. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous