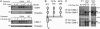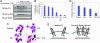Synergistic down-regulation of receptor tyrosine kinases by combinations of mAbs: implications for cancer immunotherapy - PubMed (original) (raw)
Synergistic down-regulation of receptor tyrosine kinases by combinations of mAbs: implications for cancer immunotherapy
Lilach M Friedman et al. Proc Natl Acad Sci U S A. 2005.
Abstract
mAbs to receptor tyrosine kinases such as EGF receptor/ErbB-1 and HER2/ErbB-2 inhibit the tumorigenic growth of certain cancer cells, but although recombinant versions of such Abs are already used in oncology wards, the mechanism underlying immunotherapy remains unknown. We report that anti-EGF receptor Abs promote a slow endocytic process distinct from the rapid EGF-induced receptor internalization. Combining mAbs that engage distinct epitopes significantly accelerates receptor degradation. In addition, mAb combinations are more effective than single Abs in inhibiting HER2 signaling in vitro and tumorigenesis in animals. We present a model attributing efficacy of immunotherapy to the size of Ab-receptor lattices formed at the cell surface, which dictates the rate of endocytic clearance and extent of signaling blockade.
Figures
Fig. 1.
Down-regulation of EGFR by mAbs is independent of receptor ubiquitylation. (A) KB cells were treated with EGF (100 ng/ml) or mAbs (20 μg/ml) at 37°C for various time intervals. The cells were then washed and acid-stripped, and levels of surface receptor were determined (average ± SD) by using a radiolabeled EGF. (B) KB cells were treated as indicated for 4 or 32 h and washed as in A. This treatment was followed either by immunoblotting with an anti-EGFR Ab (Lower) or by surface biotinylation, followed by immunoprecipitation and blotting with streptavidin-horseradish peroxidase (Upper). (C) CHO cells transiently expressing EGFR, c-Cbl, and hemagglutinin-ubiquitin were incubated with EGF (100 ng/ml) or the indicated mAb (20 μg/ml) for various intervals. (D) CHO cells transiently expressing WT- or Y1045F-EGFR, along with c-Cbl, were incubated with EGF (100 ng/ml; 1 h), saline, or the indicated mAbs (10 μg/ml; 18 h). EGFR was analyzed after removal of bound ligands.
Fig. 2.
Combinations of anti-receptor mAbs down-regulate EGFR better than each mAb alone. (A) KB cells were treated for 13 h with EGF (100 ng/ml) or the indicated mAbs (total: 20 μg/ml). Cell extracts were analyzed after stripping of bound ligands. (B) KB cells were treated for various time intervals and extracts analyzed as in A.(C) KB cells were treated for 1 h at 4°C with mAbs 111 (•), 143 (▴), 565 (○), or EGF (▪). The indicated radiolabeled mAbs (8 nM) were then added, and the cells were incubated for an additional 15 min before determination of radioactivity. (D Upper) KB cells were treated for 18 h with saline (Cont) or EGF (100 ng/ml) or cotreated with mAbs 111 (10 μg/ml) and 565 (2.5–20 μg/ml). (D Lower) KB cells were preincubated with saline, EGF (100 ng/ml), or the indicated mAbs (total: 10 μg/ml) for 3 h at 4°C. The medium of all cells (except lane 3) was then replaced with fresh medium containing a goat anti-mouse IgG [second Ab: either 40 (+) or 10, 20, and 40 μg/ml], and cells were incubated for additional 18 h at 37°C.
Fig. 3.
Combinations of mAbs down-regulate ErbB-2 better than each mAb alone. (A) SKBR-3 cells were treated for 1 h with various concentrations of mAbs L26 (circles) or Herceptin (triangles) at 4°C. Radiolabeled mAbs L26 (8 nM; filled symbols) or Herceptin (open symbols) were then added, and the cells were incubated for 15 min. After washing, radioactivity was measured and expressed as average ± SD. (B) HEK-293T cells (Upper) ectopically expressing ErbB-2 or T47D cells (Lower) were treated with L26 and/or Herceptin (Her; total: 20 μg/ml) at 37°C for the indicated time intervals. PI, preimmune Abs. (C) SKBR-3 cells were treated at 37°C for the indicated time intervals with fluorescein-labeled 4D5-mAb (10 μg/ml) in the absence (▴) or presence (▪) of L26-mAb. Thereafter, cells were washed and acid-stripped, and internalized 4D5 was determined by using a cell sorter. (D) CB2 cells were incubated with a mixture of L26 and Herceptin (20 μg/ml each) or L26 alone (40 μg/ml) at 37°C for the indicated time periods. Thereafter, cells were washed, fixed, and permeabilized, and ErbB-2 was detected by using confocal microscopy with a Cy3-conjugated anti-mouse IgG.
Fig. 4.
Down-regulation of ErbB-2 by combinations of mAbs is dynamin-dependent but requires no cytoplasmic or transmembrane portions of ErbB-2. (A) HEK-293T cells were cotransfected with plasmids encoding EGFR (Upper) or ErbB-2 (Lower), along with plasmids encoding c-Cbl and dynamin (WT or K44A). After 48 h, cells were treated with EGF (100 ng/ml) or a combination of L26 and Herceptin (Her; total: 20 μg/ml), and extracts were analyzed. (B) A diagram of ErbB-2 molecules analyzed, either WT, a mutant lacking the cytoplasmic domain (ECD-TM), or the full ectodomain fused to a GPI-attachment signal (ECD-GPI). (C) CHO cells were treated for 3 h with a mixture of mAbs L26 and Herceptin (5 μg/ml each). Note that only the surface-localized forms of ECD-GPI and ECD-TM (arrows) were affected by mAbs.
Fig. 5.
Combinations of anti-ErbB-2 Abs inhibit growth factor signaling, promote differentiation, and reduce tumor growth. (A) T47D cells were incubated for 12 h with mAbs (total: 10 μg/ml), including a mixture (mix) of L26 and Herceptin (Her). Cells were then washed and stimulated with NDF (50 ng/ml) for 15 min, and cell extracts were analyzed. (B) MCF-7 cells were transfected with a fos reporter plasmid, and, 24 h later, cells were split and incubated for 12 h with the indicated Abs, including a control human IgG. Later (47 h), cells were washed and stimulated for 1 h with NDF (50 ng/ml), followed by analysis by using a luminator. (C) AU-565 cells were treated for 3 d with mAbs (30 μg/ml) and then stained for neutral lipids. (D) CD-1/nude mice were injected s.c. with 3 × 106 N87 cells. mAbs (600 μg per animal) were injected i.p. 3, 7, and 10 d later. Saline-injected mice were used for control. Tumor volumes were measured after 18 d, and the mean volume of each group of four mice was plotted. The difference between treatments with each mAb alone and their combination is statistically significant (P < 0.05). (E) A model comparing the size of ErbB-Ab complexes formed at the cell surface by one or two mAbs. We propose that the rate of internalization is proportional to the size of surface-associated antigen-Ab lattices.
Similar articles
- Monoclonal antibodies directed to the erbB-2 receptor inhibit in vivo tumour cell growth.
Harwerth IM, Wels W, Schlegel J, Müller M, Hynes NE. Harwerth IM, et al. Br J Cancer. 1993 Dec;68(6):1140-5. doi: 10.1038/bjc.1993.494. Br J Cancer. 1993. PMID: 7903153 Free PMC article. - Antitumor effects of doxorubicin in combination with anti-epidermal growth factor receptor monoclonal antibodies.
Baselga J, Norton L, Masui H, Pandiella A, Coplan K, Miller WH Jr, Mendelsohn J. Baselga J, et al. J Natl Cancer Inst. 1993 Aug 18;85(16):1327-33. doi: 10.1093/jnci/85.16.1327. J Natl Cancer Inst. 1993. PMID: 8340945 - Endocytic downregulation of ErbB receptors: mechanisms and relevance in cancer.
Roepstorff K, Grøvdal L, Grandal M, Lerdrup M, van Deurs B. Roepstorff K, et al. Histochem Cell Biol. 2008 May;129(5):563-78. doi: 10.1007/s00418-008-0401-3. Epub 2008 Feb 21. Histochem Cell Biol. 2008. PMID: 18288481 Free PMC article. Review. - Type I receptor tyrosine kinases as targets for therapy in breast cancer.
Baselga J, Mendelsohn J. Baselga J, et al. J Mammary Gland Biol Neoplasia. 1997 Apr;2(2):165-74. doi: 10.1023/a:1026355831693. J Mammary Gland Biol Neoplasia. 1997. PMID: 10882302 Review.
Cited by
- An anti-HER2 biparatopic antibody that induces unique HER2 clustering and complement-dependent cytotoxicity.
Weisser NE, Sanches M, Escobar-Cabrera E, O'Toole J, Whalen E, Chan PWY, Wickman G, Abraham L, Choi K, Harbourne B, Samiotakis A, Rojas AH, Volkers G, Wong J, Atkinson CE, Baardsnes J, Worrall LJ, Browman D, Smith EE, Baichoo P, Cheng CW, Guedia J, Kang S, Mukhopadhyay A, Newhook L, Ohrn A, Raghunatha P, Zago-Schmitt M, Schrag JD, Smith J, Zwierzchowski P, Scurll JM, Fung V, Black S, Strynadka NCJ, Gold MR, Presta LG, Ng G, Dixit S. Weisser NE, et al. Nat Commun. 2023 Mar 13;14(1):1394. doi: 10.1038/s41467-023-37029-3. Nat Commun. 2023. PMID: 36914633 Free PMC article. - Regulation of focal adhesion turnover by ErbB signalling in invasive breast cancer cells.
Xu Y, Benlimame N, Su J, He Q, Alaoui-Jamali MA. Xu Y, et al. Br J Cancer. 2009 Feb 24;100(4):633-43. doi: 10.1038/sj.bjc.6604901. Epub 2009 Feb 3. Br J Cancer. 2009. PMID: 19190626 Free PMC article. - Simultaneous targeting of HER family pro-survival signaling with Pan-HER antibody mixture is highly effective in TNBC: a preclinical trial with PDXs.
Reddy TP, Choi DS, Anselme AC, Qian W, Chen W, Lantto J, Horak ID, Kragh M, Chang JC, Rosato RR. Reddy TP, et al. Breast Cancer Res. 2020 May 15;22(1):48. doi: 10.1186/s13058-020-01280-z. Breast Cancer Res. 2020. PMID: 32414394 Free PMC article. - Interaction of antibodies with ErbB receptor extracellular regions.
Schmitz KR, Ferguson KM. Schmitz KR, et al. Exp Cell Res. 2009 Feb 15;315(4):659-70. doi: 10.1016/j.yexcr.2008.10.008. Epub 2008 Oct 22. Exp Cell Res. 2009. PMID: 18992239 Free PMC article. Review. - Precision engineering of targeted nanocarriers.
Deci MB, Liu M, Dinh QT, Nguyen J. Deci MB, et al. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018 Sep;10(5):e1511. doi: 10.1002/wnan.1511. Epub 2018 Feb 13. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2018. PMID: 29436157 Free PMC article. Review.
References
- Yarden, Y. & Sliwkowski, M. X. (2001) Nat. Rev. Mol. Cell Biol. 2, 127–137. - PubMed
- Lynch, T. J., Bell, D. W., Sordella, R., Gurubhagavatula, S., Okimoto, R. A., Brannigan, B. W., Harris, P. L., Haserlat, S. M., Supko, J. G., Haluska, F. G., et al. (2004) New Engl. J. Med. 350, 2129–2139. - PubMed
- Slamon, D. J., Godolphin, W., Jones, L. A., Holt, J. A., Wong, S. G., Keith, D. E., Levin, W. J., Stuart, S. G., Udove, J., Ullrich, A., et al. (1989) Science 244, 707–712. - PubMed
- Drebin, J. A., Stern, D. F., Link, V. C., Weinberg, R. A. & Greene, M. I. (1984) Nature 312, 579–588. - PubMed
- Masui, H., Kawamoto, T., Sato, J. D., Wolf, B., Sato, G. & Mendelsohn, J. (1984) Cancer Res. 44, 1002–1007. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous