Effects of valproic acid derivatives on inositol trisphosphate depletion, teratogenicity, glycogen synthase kinase-3beta inhibition, and viral replication: a screening approach for new bipolar disorder drugs derived from the valproic acid core structure - PubMed (original) (raw)
Comparative Study
. 2005 May;67(5):1426-33.
doi: 10.1124/mol.104.009308. Epub 2005 Feb 1.
Affiliations
- PMID: 15687223
- PMCID: PMC1360212
- DOI: 10.1124/mol.104.009308
Comparative Study
Effects of valproic acid derivatives on inositol trisphosphate depletion, teratogenicity, glycogen synthase kinase-3beta inhibition, and viral replication: a screening approach for new bipolar disorder drugs derived from the valproic acid core structure
B J Eickholt et al. Mol Pharmacol. 2005 May.
Abstract
Inositol-1,4,5-trisphosphate (InsP3) depletion has been implicated in the therapeutic action of bipolar disorder drugs, including valproic acid (VPA). It is not currently known whether the effect of VPA on InsP3 depletion is related to the deleterious effects of teratogenicity or elevated viral replication, or if it occurs via putative inhibitory effects on glycogen synthase kinase-3beta (GSK-3beta). In addition, the structural requirements of VPA-related compounds to cause InsP3 depletion are unknown. In the current study, we selected a set of 10 VPA congeners to examine their effects on InsP3 depletion, in vivo teratogenic potency, HIV replication, and GSK-3beta activity in vitro. We found four compounds that function to deplete InsP3 in the model eukaryote Dictyostelium discoideum, and these drugs all cause growth-cone enlargement in mammalian primary neurons, consistent with the effect of InsP3 depletion. No relationship was found between InsP3 depletion and teratogenic or elevated viral replication effects, and none of the VPA congeners were found to affect GSK-3beta activity. Structural requirements of VPA congers to maintain InsP3 depletion efficacy greater than that of lithium are a carboxylic-acid function without dependence on side-chain length, branching, or saturation. Noteworthy is the enantiomeric differentiation if a chiral center exists, suggesting that InsP3 depletion is mediated by a stereoselective mode of action. Thus, the effect of InsP3 depletion can be separated from that of teratogenic potency and elevated viral replication effect. We have used this to identify two VPA derivatives that share the common InsP3-depleting action of VPA, lithium and carbamazepine, but do not show the side effects of VPA, thus providing promising novel candidates for bipolar disorder treatment.
Figures
Fig. 1
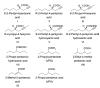
The structure of valproic acid and its congeners. Compounds are referred to by roman numeral prefix (I–IX) except for VPA and its amide derivative VPD. Compounds were chosen by broad category to result in a test set with highest structural diversity in branching (VIII), saturation (I–VI and IX), side-chain length, derivatization of the carboxylic acid function, and chiral aspects (_R_- and _S-_enantiomers: I and II, III and IV, V and VI).
Fig. 2
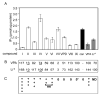
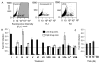
Characterization of InsP3-depletion efficacy and teratogenicity of VPA analogs and current bipolar disorder treatments. D. discoideum cells were treated with lithium, VPA, or compounds derived from the chemical structure of VPA (Fig. 1). A, cells were grown overnight in complete medium in the presence of VPA or one of its analogs at 0.5 mM, a concentration found in patient plasma undergoing VPA treatment, or with lithium at 10 mM. Changes in InsP3 levels were subsequently measured by isotope dilution assay (Amersham Biosciences UK, Ltd., Little Chalfont, Buckinghamshire, UK; see Materials and Methods), and compared with vehicle-only (▪) control or currently used bipolar disorder treatments (▧). Results represent four experiments assayed in triplicate (± S.E.M.). B and C, comparison of relative efficacy of VPA congeners to cause InsP3 depletion expressed as a percentage of that found for VPA or lithium (underline indicates increased InsP3 levels). Compounds were tested for teratogenic rating in an in vivo model. Arbitrary scale of teratogenic ratings for these drugs, from not teratogenic (0) to highly teratogenic (+++++), as described under Materials and Methods (★, inferred from Phiel et al., 2001; Gurvich et al., 2004). RM, racemic mixture; ND, not determined.
Fig. 3
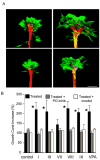
Enlargement of growth cones from DRG neurons after treatment with InsP3-depleting drugs. Rat DRGs were cultured for 24 h in the presence of 0.5 mM VPA, analogs showing InsP3 depletion in D. discoideum, or one analog showing no effect on InsP3 levels in D. discoideum (Fig. 2A), and afterward, cells were fixed and stained before growth-cone size quantification. A, representative growth cones are shown for vehicle-only control (top left), 0.5 mM VPA (top right), or VPA with _myo_-inositol (2 mM) (bottom left) or prolyl oligopeptidase inhibitor (133 μM) (bottom right). B, quantification of growth-cone size for five VPA derivatives and VPA after treatment (▪) with the indicated drug or with the drug plus inositol (□) or PO inhibitor (▧). Data represent two to four independent experiments containing approximately 25 growth cones per experiment (± S.E.M.); ★, p < 0.05.
Fig. 4
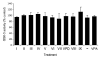
Direct inhibition studies of GSK-3β activity by VPA and its 10 congeners. Drugs (3 mM) were tested for inhibitory effect on mammalian GSK-3β with vector only control (−, dimethyl sulfoxide). Results are expressed as a percentage of activity in the absence of drug ± S.D.
Fig. 5
Characterization of bipolar disorder treatments, VPA analogs, and other treatments of HIV replication. Human 293T cells were infected with GFP-labeled HIV particles modified to block cell lysis in the presence of the indicated drugs. Cells were analyzed for the presence of GFP-labeled HIV after 48 h using FACS analysis (see Materials and Methods). A, cell infection rates were quantified by measuring GFP-labeled cells, shown for control (1.1%), compound IX (1.1%), and VPA (2.5%) treatments with increased FL1 levels (gray shaded area, control). Quantification of HIV infection is provided as increase in infection rates over control (vehicle only) for B. B, the indicated compounds at low (0.5 mM) and high concentrations (3 mM) or lithium (3 and 10 mM) or an inhibitor of GABA transaminases (VGB, vigabatrin: 30 and 100 μM). C, the inhibition of histone deacetylases by VPA was mimicked using a range of concentrations of trichostatin A (TSA). Decreased infection rates reflect toxicity of the compounds on target cells at high drug concentrations. Data represent FACS analysis of at least two independent experiments (± S.E.M.).
Similar articles
- Synergistic neuroprotective effects of lithium and valproic acid or other histone deacetylase inhibitors in neurons: roles of glycogen synthase kinase-3 inhibition.
Leng Y, Liang MH, Ren M, Marinova Z, Leeds P, Chuang DM. Leng Y, et al. J Neurosci. 2008 Mar 5;28(10):2576-88. doi: 10.1523/JNEUROSCI.5467-07.2008. J Neurosci. 2008. PMID: 18322101 Free PMC article. - Valproic Acid Modifies Synaptic Structure and Accelerates Neurite Outgrowth Via the Glycogen Synthase Kinase-3β Signaling Pathway in an Alzheimer's Disease Model.
Long ZM, Zhao L, Jiang R, Wang KJ, Luo SF, Zheng M, Li XF, He GQ. Long ZM, et al. CNS Neurosci Ther. 2015 Nov;21(11):887-97. doi: 10.1111/cns.12445. Epub 2015 Sep 19. CNS Neurosci Ther. 2015. PMID: 26385876 Free PMC article. - The effects of central nervous system-active valproic acid constitutional isomers, cyclopropyl analogs, and amide derivatives on neuronal growth cone behavior.
Shimshoni JA, Dalton EC, Jenkins A, Eyal S, Ewan K, Williams RS, Pessah N, Yagen B, Harwood AJ, Bialer M. Shimshoni JA, et al. Mol Pharmacol. 2007 Mar;71(3):884-92. doi: 10.1124/mol.106.030601. Epub 2006 Dec 13. Mol Pharmacol. 2007. PMID: 17167030 - Structure-function studies for the panacea, valproic acid.
Terbach N, Williams RS. Terbach N, et al. Biochem Soc Trans. 2009 Oct;37(Pt 5):1126-32. doi: 10.1042/BST0371126. Biochem Soc Trans. 2009. PMID: 19754465 Review. - Search for a common mechanism of mood stabilizers.
Harwood AJ, Agam G. Harwood AJ, et al. Biochem Pharmacol. 2003 Jul 15;66(2):179-89. doi: 10.1016/s0006-2952(03)00187-4. Biochem Pharmacol. 2003. PMID: 12826261 Review.
Cited by
- The dictyostelium kinome--analysis of the protein kinases from a simple model organism.
Goldberg JM, Manning G, Liu A, Fey P, Pilcher KE, Xu Y, Smith JL. Goldberg JM, et al. PLoS Genet. 2006 Mar;2(3):e38. doi: 10.1371/journal.pgen.0020038. Epub 2006 Mar 31. PLoS Genet. 2006. PMID: 16596165 Free PMC article. - Valproic acid inhibits Abeta production, neuritic plaque formation, and behavioral deficits in Alzheimer's disease mouse models.
Qing H, He G, Ly PT, Fox CJ, Staufenbiel M, Cai F, Zhang Z, Wei S, Sun X, Chen CH, Zhou W, Wang K, Song W. Qing H, et al. J Exp Med. 2008 Nov 24;205(12):2781-9. doi: 10.1084/jem.20081588. Epub 2008 Oct 27. J Exp Med. 2008. PMID: 18955571 Free PMC article. - GSK-3 is a viable potential target for therapeutic intervention in bipolar disorder.
Rowe MK, Wiest C, Chuang DM. Rowe MK, et al. Neurosci Biobehav Rev. 2007;31(6):920-31. doi: 10.1016/j.neubiorev.2007.03.002. Epub 2007 Mar 15. Neurosci Biobehav Rev. 2007. PMID: 17499358 Free PMC article. Review. - Attenuation of phospholipid signaling provides a novel mechanism for the action of valproic acid.
Xu X, Müller-Taubenberger A, Adley KE, Pawolleck N, Lee VW, Wiedemann C, Sihra TS, Maniak M, Jin T, Williams RS. Xu X, et al. Eukaryot Cell. 2007 Jun;6(6):899-906. doi: 10.1128/EC.00104-06. Epub 2007 Apr 13. Eukaryot Cell. 2007. PMID: 17435006 Free PMC article. - Inositol synthesis regulates the activation of GSK-3α in neuronal cells.
Ye C, Greenberg ML. Ye C, et al. J Neurochem. 2015 Apr;133(2):273-83. doi: 10.1111/jnc.12978. Epub 2014 Nov 17. J Neurochem. 2015. PMID: 25345501 Free PMC article.
References
- Berridge MJ, Downes CP, Hanley MR. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989;59:411–419. - PubMed
- Bojic U, Ehlers K, Ellerbeck U, Bacon CL, O’Driscoll E, O’Connell C, Berezin V, Kawa A, Lepekhin E, Bock E, et al. Studies on the teratogen pharmacophore of valproic acid analogues: evidence of interactions at a hydrophobic centre. Eur J Pharmacol. 1998;354:289–299. - PubMed
- Bojic U, Elmazar MMA, Hauck RS, Nau H. Further branching of valproate-related carboxylic acids reduces the teratogenic activity, but not the anticonvulsant effect. Chem Res Toxicol. 1996;9:866–870. - PubMed
- Breen G, Harwood AJ, Gregory K, Sinclair M, Collier D, St Clair D, Williams RS. Two peptidase activities decrease in treated bipolar disorder not schizophrenic patients. Bipolar Disord. 2004;6:156–161. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous