Identification and characterization of circulating human transitional B cells - PubMed (original) (raw)
Identification and characterization of circulating human transitional B cells
Gary P Sims et al. Blood. 2005.
Abstract
Murine B-cell development begins in bone marrow and results in the generation of immature transitional B cells that transit to the spleen to complete their maturation. It remains unclear whether the same developmental pathway takes place in humans. Using markers characteristic of human bone marrow immature B cells, we have identified a population of circulating human B cells with a phenotype most similar to mouse transitional type I (T1) B cells, although these human counterparts express CD5. These cells die rapidly in culture, and B-cell activation factor member of the tumor necrosis factor (TNF) family (BAFF) does not effect their survival regardless of B-cell receptor (BCR) stimulation. In contrast, bone marrow stromal cells or interleukin-4 (IL-4) significantly enhanced their survival. In the presence of T-cell signals provided by IL-4 or CD40 ligation, BCR stimulation can induce progression into cell cycle. Interestingly, circulating B cells that phenotypically and functionally resemble murine T2 B cells are found in cord blood and adult peripheral blood, suggesting that B-cell maturation may not be restricted to the spleen. Notably, increased proportions of T1 B cells were found in blood of patients with systemic lupus erythematosus (SLE), although bone marrow production and selection appeared to be normal.
Figures
Figure 1.
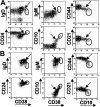
Immature B-cell markers expressed by adult bone marrow and term cord-blood B cells. (A) Bone marrow CD19+ B cells were gated into CD21- immature and CD21+ mature fractions and the differential expression of CD10, CD38, CD44, and CD24 was assessed relative to isotype matched control mAb (Con Ig). (B) Bone marrow CD19+ B cells were gated into CD38neg/lo mature B cells and a CD38hi immature subset. A fraction of the latter express CD20 more densely and both IgM and IgD. (C) Differential expression of CD5 and CD10 by bone marrow IgD- CD38hi pro/pre B cells and IgD+ CD38hi immature B cells. (D) Cord blood CD19+ B cells were gated into CD21neg/lo and CD21+ fractions, and the differential expression of immature bone marrow markers was assessed. Results are representative of 2 bone marrow aspirates and 10 cord blood samples.
Figure 2.
Circulating IgD+ CD38hi B cells express immature B-cell markers with an overall phenotype similar to transitional type I (T1) B cells. B cells were enriched from blood of healthy adult donors and stained with anti-CD19, anti-IgD, anti-CD38, and a fourth anti–human mAb. The differential expression of a variety of B-cell markers was compared between the IgD+ CD38hi immature population (solid line) and IgD+ CD38+ mature naive B cells (dashed line, indicated with arrow). Results are representative of data from at least 4 healthy adult donors.
Figure 3.
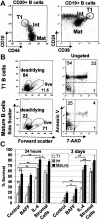
Peripheral-blood IgD+ CD38hi T1 B cells are not circulating pre–germinal-center or plasma-cell precursors. (A) Tonsil mononuclear cells were stained with anti-CD19, anti-IgD, anti-CD38, and a fourth mAb to examine the phenotype of IgD+ CD38hi pre–germinal center B cells. (B) Enriched B cells from peripheral blood of a healthy donor were similarly stained to examine the expression of plasma cell markers on CD19+ IgD+ CD38hi T1 B cells (solid gate) and CD19lo IgD- CD38bright circulating plasma cells (dashed gate). (C) The relative frequencies of the CD19+ IgD+ CD38hi T1 B-cell population (solid gate) and CD19lo IgD- CD38bright plasma cells (dashed gate) from a healthy donor were examined before immunization and 4, 7, and 14 days after immunization with influenza vaccine.
Figure 4.
T1 B cells are found in increased proportions in term cord blood and systemic lupus erythematosus patients. (A) The relative frequency of T1 B cells was determined for term cord blood (n=10), and the peripheral blood of healthy adult donors (n=29) and patients with systemic lupus erythematosus (SLE; n=18). Horizontal bars indicate means. Significant differences using the nonparametric Mann-Whitney U test were detected at P <.01 (**) and P <.001(***). (B) A linear regression plot shows that the frequency of B cells with a T1 B-cell phenotype is inversely proportional to the absolute number of peripheral lymphocytes.
Figure 5.
“Intermediate” B cells are present in cord and peripheral blood. B cells from (A) peripheral and (B) cord blood were stained with various combinations of immature and T1 B-cell markers. The T1 population (solid gate), naive B cells (dashed gate), and “intermediate B cells” (arrow) are indicated.
Figure 6.
T1 B cells are short-lived in culture although survival can be improved by coculture with mouse bone marrow stromal cells or IL-4 but not BAFF. (A) Negatively selected peripheral blood B cells from healthy adult donors were stained with anti-CD20, anti-CD10, and anti-CD44 or anti-CD19, anti-CD24, and anti-CD38. T1 B cell (CD20hi CD10hi CD44lo or CD19+ CD24hi CD38hi), mature naive (Mat) (CD20+ CD10- CD44hi or CD19+, CD24lo, CD38lo) and intermediate (Int) (CD20+ CD10lo CD44hi or CD19+ CD24int, CD38int) B-cell populations were sorted as shown. Postsorting analysis indicated that each population was more than 95% pure. (B) T1 and mature B-cell populations were cultured for 24 hours and stained with annexin V and 7-AAD. (C) T1 B cells, intermediate and mature B cell populations were cultured in medium alone or with the addition of either BAFF (200 ng/mL), IL-4 (100 ng/mL), or cultured on mouse S13 bone marrow stromal cells. After 24 hours or 3 days in culture, the cells were examined for viability by flow cytometry using forward/side scatter characteristics or 7-AAD exclusion. The data show the means ± SEM of at least 4 independent experiments. Unpaired t tests were used to detect significant differences (*P <.05; **P <.01).
Figure 7.
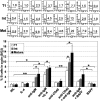
T1 B cells have reduced capacity to enter cell cycle following B-cell receptor engagement. T1, mature, and intermediate B-cell populations (Figure 4A) were cultured alone (NIL) or stimulated with various combinations of IL-4 (100 ng/mL), anti-IgM (10 μg/mL), anti-CD40 (1 μg/mL), or BAFF (200 ng/mL) for 48 hours. Afterward, hypotonic propidium iodide staining was carried out to determine apoptotic and cycling cells. Forward/side scatter and FL2 width gating were used to gate out nuclear fragments and doublets. The FL2 area was examined to identify the frequency of sub G0/G1 apoptotic cells (left number) and S/G2 cycling cells (right number). A representative experiment is shown in the top panel, and the means ± SEM of 4 independent experiments is shown in the bottom panel. Unpaired t tests were used to detect significant differences (*P <.05; **P <.01).
Similar articles
- BAFF is a survival and maturation factor for mouse B cells.
Rolink AG, Tschopp J, Schneider P, Melchers F. Rolink AG, et al. Eur J Immunol. 2002 Jul;32(7):2004-10. doi: 10.1002/1521-4141(200207)32:7<2004::AID-IMMU2004>3.0.CO;2-5. Eur J Immunol. 2002. PMID: 12115621 - BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway.
Craxton A, Draves KE, Gruppi A, Clark EA. Craxton A, et al. J Exp Med. 2005 Nov 21;202(10):1363-74. doi: 10.1084/jem.20051283. J Exp Med. 2005. PMID: 16301744 Free PMC article. - Positive and negative cooperativity of TNF and Interferon-γ in regulating synovial fibroblast function and B cell survival in fibroblast/B cell co-cultures.
Lowin T, Anssar TM, Bäuml M, Classen T, Schneider M, Pongratz G. Lowin T, et al. Sci Rep. 2020 Jan 21;10(1):780. doi: 10.1038/s41598-020-57772-7. Sci Rep. 2020. PMID: 31964950 Free PMC article. - [The role of BAFF in autoimmune diseases].
Matsushita T, Sato S. Matsushita T, et al. Nihon Rinsho Meneki Gakkai Kaishi. 2005 Oct;28(5):333-42. doi: 10.2177/jsci.28.333. Nihon Rinsho Meneki Gakkai Kaishi. 2005. PMID: 16276047 Review. Japanese. - Actions of BAFF in B cell maturation and its effects on the development of autoimmune disease.
Melchers F. Melchers F. Ann Rheum Dis. 2003 Nov;62 Suppl 2(Suppl 2):ii25-7. doi: 10.1136/ard.62.suppl_2.ii25. Ann Rheum Dis. 2003. PMID: 14532143 Free PMC article. Review.
Cited by
- HTJoinSolver: Human immunoglobulin VDJ partitioning using approximate dynamic programming constrained by conserved motifs.
Russ DE, Ho KY, Longo NS. Russ DE, et al. BMC Bioinformatics. 2015 May 23;16(1):170. doi: 10.1186/s12859-015-0589-x. BMC Bioinformatics. 2015. PMID: 26001675 Free PMC article. - Development of a Modular Assay for Detailed Immunophenotyping of Peripheral Human Whole Blood Samples by Multicolor Flow Cytometry.
Rühle PF, Fietkau R, Gaipl US, Frey B. Rühle PF, et al. Int J Mol Sci. 2016 Aug 11;17(8):1316. doi: 10.3390/ijms17081316. Int J Mol Sci. 2016. PMID: 27529227 Free PMC article. - B cells and autoimmunity.
Pillai S, Mattoo H, Cariappa A. Pillai S, et al. Curr Opin Immunol. 2011 Dec;23(6):721-31. doi: 10.1016/j.coi.2011.10.007. Epub 2011 Nov 24. Curr Opin Immunol. 2011. PMID: 22119110 Free PMC article. Review. - Mass cytometry analysis reveals attrition of naïve and anergized self-reactive non-malignant B cells in chronic lymphocytic leukemia patients.
Andrieu T, Mondière P, Jouve PE, Dussurgey S, Malassigné V, Servanton H, Baseggio L, Davi F, Michallet AS, Defrance T. Andrieu T, et al. Front Oncol. 2022 Oct 31;12:1020740. doi: 10.3389/fonc.2022.1020740. eCollection 2022. Front Oncol. 2022. PMID: 36387187 Free PMC article. - B lymphocyte-typing for prediction of clinical response to rituximab.
Brezinschek HP, Rainer F, Brickmann K, Graninger WB. Brezinschek HP, et al. Arthritis Res Ther. 2012 Jul 6;14(4):R161. doi: 10.1186/ar3901. Arthritis Res Ther. 2012. PMID: 22770118 Free PMC article.
References
- Hartley SB, Crosbie J, Brink R, Kantor AB, Basten A, Goodnow CC. Elimination from peripheral lymphoid-tissues of self-reactive lymphocytes-B recognizing membrane-bound antigens. Nature. 1991;353: 765-769. - PubMed
- Nemazee DA, Burki K. Clonal deletion of lymphocyte-B in a transgenic mouse bearing anti-Mhc class-I antibody genes. Nature. 1989;337: 562-566. - PubMed
- Norvell A, Mandik L, Monroe JG. Engagement of the antigen-receptor on immature murine B-lymphocytes results in death by apoptosis. J Immunol. 1995;154: 4404-4413. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials