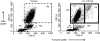Cross-linking of neutrophil CD11b results in rapid cell surface expression of molecules required for antigen presentation and T-cell activation - PubMed (original) (raw)
Cross-linking of neutrophil CD11b results in rapid cell surface expression of molecules required for antigen presentation and T-cell activation
Gavin P Sandilands et al. Immunology. 2005 Mar.
Abstract
Recent studies suggest that neutrophils may play a role in antigen presentation. In support of this hypothesis it has been shown that these cells appear to contain cytoplasmic stores of molecules required for this function, i.e. major histocompatibility complex class II (DR) antigen, CD80 and CD86. In this study we have considered a mechanism for the translocation of these preformed molecules onto the cell surface which does not require active synthesis. Cross-linking of the Mac-1 molecule (CD18 + CD11b) was shown to result in rapid cell surface expression of CD80, CD86 and DR antigen on the surface of normal human peripheral blood neutrophils. A distinct subpopulation (approximately 20%) of neutrophils appeared to be enlarged and were found to express significantly elevated levels of these molecules on the cell surface following cross-linking of CD11b when compared with control cells. The level of expression of CD80, CD86 and DR antigen on these large cells was comparable to, and in some cases greater than, the levels found expressed on the surface of monocytes obtained from the same donors. In addition, these cytoplasmic molecules were shown by confocal laser microscopy and by immunoelectron microscopy to be located within secretory vesicles. Following rapid translocation onto the cell surface, CD80 and CD86 appeared to be colocalized within large clusters reminiscent of the supramolecular antigen clusters previously found on conventional antigen-presenting cells. These findings therefore lend further support for the hypothesis that neutrophils may have a role to play in antigen presentation and/or T-cell activation.
Figures
Figure 1
Effect of cross-linking CD11b on light scatter properties of normal human peripheral blood leucocytes. Dot-plots of forward scatter (cell size) versus side scatter (granularity) showing three main populations of leucocytes before (Control) and following in vitro cross-linking of CD11b (X-L CD11b). Lymphocytes were gated as region 1 (R1), monocytes gated as Region 2 (R2) and neutrophils as region 3 (R3). For the purposes of analysis, neutrophils were further subdivided into two main populations, i.e. P1, corresponding to size and granularity of control neutrophils and larger P2 cells which appear only following cross-linking of CD11b. The P2 gate was set according to the upper limit for forward scatter of control cells to ensure that all cells appearing in the P2 region were a direct result of cross-linking. In this example, cells were incubated at 37° for 30 min in cross-linking reagent: Goat F(ab′)2 anti-mouse IgG.
Figure 2
Morphological appearance of leucocytes following in vitro cross-linking of CD11b. Cytospin preparations stained with haematoxylin and eosin of leucocytes before (control) and after cross-linking of CD11b (X-L CD11b) at 37° for 30 min. Some individual neutrophils appear larger than normal with a voluminous cytoplasm as indicated by arrows.
Figure 3
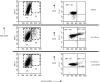
Effect of cross-linking CD11b on surface expression of CD18. Dot-plots of forward scatter (cell size) versus side scatter (granularity) showing the two main populations of neutrophils before (Control) and following in vitro cross-linking of CD11b (X-L CD11b) conducted in the absence or presence of 2 m
m
EDTA. Large P2 neutrophils appear only following cross linking of CD11b. The total number of cells appearing in the P2 region is diminished in the presence of EDTA. Dot-plots of forward scatter versus log fluorescence (CD18) show that virtually all neutrophils constitutively express CD18 on the cell surface. The mean fluorescence intensity of binding of FITC-conjugated anti-CD18 increased approximately fivefold on the surface of P1 neutrophils and by 10-fold on P2 cells following cross linking of CD11b at 37° for 30 min. When cross-linking was conducted in the presence of 2 m
m
EDTA then the MFI value for CD18 expression was essentially normal.
Figure 4
Effect of cross-linking CD11b on neutrophil surface expression of CD66, CD64, CD80, CD86 and MHC class II (DR) antigen: Whole blood method. Dot-plots of forward scatter versus log fluorescence for all neutrophils gated as R3 (see Fig. 1) are shown for control samples (no cross-linking) and following in vitro cross-linking of CD11b (X-L CD11b) at 37° for 30 min. This figure shows the results of a typical experiment performed using whole blood obtained from one individual donor. Surface expression of CD antigens was measured relative to an appropriate isotype matched mouse IgG isotype control (mIgG1). Quadrant gates were set according to mouse IgG negative control values in each experiment. The horizontal line indicates the upper limit for binding of mouse IgG1 and the vertical line indicates the forward scatter gate placed at the upper limit for normal control neutrophils. The percentage of cells appearing in the upper quadrants (positive cells) is shown for comparison and the mean fluorescence intensity is also indicated in brackets. Cells appearing in the upper left quadrant are therefore P1 neutrophils and the upper right quadrant are P2 cells.
Figure 5
Effect of cross-linking CD11b on neutrophil surface expression of CD66, CD64, CD80, CD86 and MHC class II (DR) antigen: Purified neutrophils. In order to provide a valid comparison, this assay was performed using the same donor, on the same day, in parallel with the whole blood method as shown above in Fig. 4. Neutrophils were purified using Ficoll-hypaque, dextran sedimentation and hypotonic shock. (a) For analysis, purified neutrophils were gated as indicated by the region shown using dot-plots of forward versus side scatter. (b) Dot-plots of forward scatter versus log fluorescence for all neutrophils are shown for control samples (no cross-linking) and following in vitro cross-linking of CD11b (X-L CD11b) at 37° for 30 min. Surface expression of CD antigens was measured relative to an appropriate isotype-matched mouse IgG isotype control (mIgG1). Quadrant gates were set according to the mouse IgG negative control for the cross-linked preparations. The horizontal line indicates the upper limit for binding of mouse IgG1 and the vertical line indicates the point at which large P2 cells appear. The percentage of cells appearing in the upper quadrants (positive cells) is shown for comparison and the mean fluorescence intensity is also indicated in brackets. Cells appearing in the upper left quadrant are therefore P1 neutrophils and the upper right quadrant are P2 cells.
Figure 6
Detection of cytoplasmic CD80 by flow-cytometry following fixation and permeabilization of normal human peripheral blood neutrophils: Dual-staining using granule markers. Dot-plots of log fluorescence for FITC (_X_-axis) versus R-PE (_Y_-axis) before (Control) and following fixation and permeabilization (Permeabilized) of neutrophils, i.e. cells gated as region 3 – see Fig. 1. Dot-plots of control cells show that monoclonal antibodies specific for CD80 and those specific for the four different types of neutrophil granule, i.e. myeloperoxidase (azurophilic granules), lactoferrin (specific granules), gelatinase (tertiary granules) and human serum albumin (secretory vesicles) do not bind to the surface of neutrophils. In contrast, following fixation and permeabilization, all neutrophils show dual staining.
Figure 7
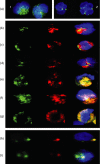
(a-g): Detection of cytoplasmic CD80 by confocal microscopy following fixation and permeabilization of normal human peripheral blood neutrophils: Dual-staining using various granule markers.(a) Confocal image of twopermeabilized neutrophils showing that binding of FITC-conjugated (green) anti-CD80(1) and CD86(2) appears to be confined to granules within the cell cytoplasm. Cell nucleus counterstained with 0.2 mg/ml 40′,6-diamidino-2-phenylindole (DAPI). Each individual cell shown in this figure was approximately 10 ;Cm in diameter. (b) Confocal image of a single permeabilized neutrophil showing green (FITC) alone (CD80), red (R-PE) alone (myeloperoxidase) and the merged image + DAPI. Red and green areas are distinct indicating that CD80 is not located within primary (azurophilic) granules. (c) Confocal image of a single permeabilized neutrophil showing green (FITC) alone (CD80), red (R-PE) alone (lactoferrin) and the merged image + DAPI. Red and green areas are distinct indicating that CD80 is not located within secondary (specific) granules. (d) Confocal image of a single permeabilized neutrophil showing green (FITC) alone (CD80), red (R-PE) alone (MMP-9) and the merged image + DAPI. Red and green areas are distinct indicating that CD80 is not located within tertiary (gelatinase) granules. (e) Confocal image of a single permeabilized neutrophil showing green (FITC) alone (human serum albumin), red (R-PE) alone (CD80) and the merged image + DAPI. Red and green areas are identical indicating that CD80 is located within secretory vesicles. (f) Confocal image of a single permeabilized neutrophil showing green (FITC) alone (CD80), red (R-PE) alone (MHC class II [DR] antigen) and the merged image + DAPI. Red and green areas are identical indicating that these molecules are colocalized within secretory vesicles. (g) Confocal image of a single permeabilized neutrophil showing green (FITC) alone (CD80), red (R-PE) alone (CD86) and the merged image + DAPI. Red and green areas are identical indicating that these molecules are colocalized within secretory vesicles. (h-i) Detection of cell surface CD80 and CD86 following cross-linking of CD11bh. Confocal image of a single neutrophil following cross-linking of CD11b at 37° for 2·5 min showing green (FITC) alone (CD80), red (R-PE) alone (CD86) and the merged image + DAPI. Red and green areas are identical indicating that these molecules are colocalized on the cell surface within a single large cluster. Different z-levels taken through this particular cell confirmed that this was the only area on the surface where these molecules were found to be colocalized. (i) Confocal image of a single neutrophil following cross-linking of CD11b at 37° for 90 min showing green (FITC) alone (CD80), red (R-PE) alone (CD86) and the merged image + DAPI. Two distinct clusters were observed on the surface of this cell. In one cluster only CD80 (FITC) was observed while the other cluster clearly contains both molecules as indicated by the yellow colour in the merged image. Note: In this study many hundreds of confocal images were obtained. For practical reasons the images shown in this figure were therefore selected to illustrate the authors conclusions based on detailed inspection of multiple images.
Figure 8
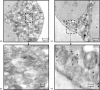
Detection of cytoplasmic CD80 by immunoelectron microscopy using 10 nm gold particles conjugated with F(ab′)2 goat anti-mouse IgG. Pre-embedding method. (a, b) Low and high power transmission electron micrographs showing part of a control neutrophil, were primary antibody (mouse anti CD80) was omitted showing a few individual gold particles within the cytoplasm. Clusters of particles were never observed in these preparations. (c, d) Low and high power transmission electron micrographs showing part of a neutrophil, were primary antibody (mouse anti-CD80) was included showing definite clustering (see arrows) of particles within the cell cytoplasm possibly associated with small vesicle like structures within the cell cytoplasm. Immunogold particles did not bind to the cell nucleus nor were they ever found to be associated with large (primary and/or secondary) granules.
Similar articles
- Major histocompatibility complex class II (DR) antigen and costimulatory molecules on in vitro and in vivo activated human polymorphonuclear neutrophils.
Sandilands GP, McCrae J, Hill K, Perry M, Baxter D. Sandilands GP, et al. Immunology. 2006 Dec;119(4):562-71. doi: 10.1111/j.1365-2567.2006.02471.x. Epub 2006 Oct 11. Immunology. 2006. PMID: 17034427 Free PMC article. - Expression of molecules involved in antigen presentation and T cell activation (HLA-DR, CD80, CD86, CD44 and CD54) by cultured human osteoblasts.
Reyes-Botella C, Montes MJ, Vallecillo-Capilla MF, Olivares EG, Ruiz C. Reyes-Botella C, et al. J Periodontol. 2000 Apr;71(4):614-7. doi: 10.1902/jop.2000.71.4.614. J Periodontol. 2000. PMID: 10807126 - Detection of cytoplasmic CD antigens within normal human peripheral blood leucocytes.
Sandilands GP, Hauffe B, Loudon E, Marsh AG, Gondowidjojo A, Campbell C, Ferrier RK, Rodie ME. Sandilands GP, et al. Immunology. 2003 Mar;108(3):329-37. doi: 10.1046/j.1365-2567.2003.01591.x. Immunology. 2003. PMID: 12603599 Free PMC article. - T cells as antigen-presenting cells.
Pichler WJ, Wyss-Coray T. Pichler WJ, et al. Immunol Today. 1994 Jul;15(7):312-5. doi: 10.1016/0167-5699(94)90078-7. Immunol Today. 1994. PMID: 7522009 Review. - Surface antigen changes during normal neutrophilic development: a critical review.
Elghetany MT. Elghetany MT. Blood Cells Mol Dis. 2002 Mar-Apr;28(2):260-74. doi: 10.1006/bcmd.2002.0513. Blood Cells Mol Dis. 2002. PMID: 12064921 Review.
Cited by
- Granulocytes: New Members of the Antigen-Presenting Cell Family.
Lin A, Loré K. Lin A, et al. Front Immunol. 2017 Dec 11;8:1781. doi: 10.3389/fimmu.2017.01781. eCollection 2017. Front Immunol. 2017. PMID: 29321780 Free PMC article. Review. - Neutrophils in secondary lymphoid organs.
Lok LSC, Clatworthy MR. Lok LSC, et al. Immunology. 2021 Dec;164(4):677-688. doi: 10.1111/imm.13406. Epub 2021 Aug 30. Immunology. 2021. PMID: 34411302 Free PMC article. Review. - Neutrophils: a Central Point of Interaction Between Immune Cells and Nonimmune Cells in Rheumatoid Arthritis.
Wang Z, Jiao Y, Diao W, Shi T, Geng Q, Wen C, Xu J, Deng T, Li X, Zhao L, Gu J, Deng T, Xiao C. Wang Z, et al. Clin Rev Allergy Immunol. 2025 Mar 28;68(1):34. doi: 10.1007/s12016-025-09044-3. Clin Rev Allergy Immunol. 2025. PMID: 40148714 Review. - Cowpea Mosaic Virus Promotes Anti-Tumor Activity and Immune Memory in a Mouse Ovarian Tumor Model.
Wang C, Fiering SN, Steinmetz NF. Wang C, et al. Adv Ther (Weinh). 2019 May;2(5):1900003. doi: 10.1002/adtp.201900003. Epub 2019 Feb 25. Adv Ther (Weinh). 2019. PMID: 33969181 Free PMC article. - Neutrophils in Type 1 Diabetes: Untangling the Intricate Web of Pathways and Hypothesis.
Nigi L, Pedace E, Dotta F, Sebastiani G. Nigi L, et al. Biomolecules. 2025 Mar 31;15(4):505. doi: 10.3390/biom15040505. Biomolecules. 2025. PMID: 40305198 Free PMC article. Review.
References
- Cassatella MA. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–6. - PubMed
- Gosselin EJ, Wardwell K, Rigby WFC, Guyre P. Induction of MHC class II on human polymorphonucler neutrophils by granulocyte/macrophage colony-stimulating factor, IFN-γ, and IL-3. J Immunol. 1993;151:1482–90. - PubMed
- Cross A, Bucknall RC, Cassatella MA, Edwards SW, Moots RJ. Synovial fluid neutrophils transcribe and express class II major histocompatibility complex molecules in rheumatoid arthritis. Arthritis Rheum. 2003;48(10):2796–806. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous