Activation mutations of human c-KIT resistant to imatinib mesylate are sensitive to the tyrosine kinase inhibitor PKC412 - PubMed (original) (raw)
Activation mutations of human c-KIT resistant to imatinib mesylate are sensitive to the tyrosine kinase inhibitor PKC412
Joseph D Growney et al. Blood. 2005.
Abstract
Constitutively activated forms of the transmembrane receptor tyrosine kinase c-KIT have been associated with systemic mast cell disease, acute myeloid leukemia, and gastrointestinal stromal tumors. Reports of the resistance of the kinase domain mutation D816V to the adenosine triphosphate (ATP)-competitive kinase inhibitor imatinib mesylate prompted us to characterize 14 c-KIT mutations reported in association with human hematologic malignancies for transforming activity in the murine hematopoietic cell line Ba/F3 and for sensitivity to the tyrosine kinase inhibitor PKC412. Ten of 14 c-KIT mutations conferred interleukin 3 (IL-3)-independent growth. c-KIT D816Y and D816V transformed cells were sensitive to PKC412 despite resistance to imatinib mesylate. In these cells, PKC412, but not imatinib mesylate, inhibited autophosphorylation of c-KIT and activation of downstream effectors signal transducer and transcriptional activator 5 (Stat5) and Stat3. Variable sensitivities to PKC412 or imatinib mesylate were observed among other mutants. These findings suggest that PKC412 may be a useful therapeutic agent for c-KIT-positive malignancies harboring the imatinib mesylate-resistant D816V or D816Y activation mutations.
Figures
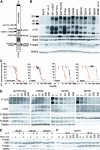
Figure 1.
Imatinib mesylate resistant c-Kit mutations are sensitive to PKC412. (A) Schematic of the c-KIT protein indicating the relative location of structural and functional domains, as well as location of 14 c-KIT mutations evaluated. The JM delVV mutation occurs at V559V560. ▪ indicates amino terminal signal peptide. Other domains of c-KIT are indicated as follows: □, extracellular domain; TM, transmembrane domain; JM, juxtamembrane domain; TK1, nucleotide binding subdomain of split kinase domain; KI, kinase insert domain; and TK2, catalytic subdomain of split kinase domain. (B) Ba/F3-transforming c-KIT mutations are constitutively phosphorylated and phosphorylate Stat3 and Stat5. Blots from top to bottom: antiphosphotyrosine Western blot of Ba/F3 lysates immunoprecipitated with anti-c-KIT; anti-c-KIT Western blot of immunoprecipitates in top blot; anti-phospho-Stat3 Western blot of whole-cell lysates (WCLs) from IP in top blot; anti-Stat3 Western blot of same WCLs; anti-phospho-Stat5 Western blot of WCLs from IP in top blot; and anti-Stat5a Western blot of the same WCLs. (C) Representative growth curves of delTYD + RG, V560G, D816Y, and D816V transduced Ba/F3 cell lines. Plotted is the percentage ± SD of 3H-thymidine incorporation of drug-treated cells relative to no-drug controls. Cells were treated with imatinib mesylate (black) or PKC412 (red). Cells were grown in the absence of IL-3 or rhSCF. (D) Dose response of PKC412 inhibition of c-KIT, Stat5, and Stat3 phosphorylation. Shown are data from delTYD + RG, V560G, D816Y, and D816V transformed Ba/F3 cells. Blots are as in panel B. Cells were grown in the absence of IL-3 and the indicated concentration of PKC412 (nM) for 4 hours. (E) PKC412 does not inhibit IL-3-induced Stat5 phosphorylation. Blots from top to bottom: anti-phospho-Stat3 Western blot of WCLs from indicated cell lines with or without IL-3 (10 ng/mL) and PKC412 (250 nM/mL), for which Ba/F3 cells were starved of IL-3 and serum for 4 hours prior to addition of IL-3 and PKC412; anti-Stat3 Western blot of WCLs in top blot; anti-phospho-Stat5 Western blot of WCLs as in top blot; and anti-Stat5a Western blot of same WCLs. (F) Dose response of Stat5 phosphorylation to PKC412 in Ba/F3 cells. Blots are as for panel E. Ba/F3 cells were starved of IL-3 and FCS for 4 hours, then incubated with or without IL-3 (10 ng/mL) in the presence of the indicated concentration of PKC412 for 4 hours.
Similar articles
- Resistance to c-KIT kinase inhibitors conferred by V654A mutation.
Roberts KG, Odell AF, Byrnes EM, Baleato RM, Griffith R, Lyons AB, Ashman LK. Roberts KG, et al. Mol Cancer Ther. 2007 Mar;6(3):1159-66. doi: 10.1158/1535-7163.MCT-06-0641. Mol Cancer Ther. 2007. PMID: 17363509 - Mechanisms of resistance to imatinib mesylate in gastrointestinal stromal tumors and activity of the PKC412 inhibitor against imatinib-resistant mutants.
Debiec-Rychter M, Cools J, Dumez H, Sciot R, Stul M, Mentens N, Vranckx H, Wasag B, Prenen H, Roesel J, Hagemeijer A, Van Oosterom A, Marynen P. Debiec-Rychter M, et al. Gastroenterology. 2005 Feb;128(2):270-9. doi: 10.1053/j.gastro.2004.11.020. Gastroenterology. 2005. PMID: 15685537 - Imatinib mesylate: in the treatment of gastrointestinal stromal tumours.
Croom KF, Perry CM. Croom KF, et al. Drugs. 2003;63(5):513-22; discussion 523-4. doi: 10.2165/00003495-200363050-00005. Drugs. 2003. PMID: 12600228 Review. - Targeting c-kit mutations in solid tumors: scientific rationale and novel therapeutic options.
Demetri GD. Demetri GD. Semin Oncol. 2001 Oct;28(5 Suppl 17):19-26. Semin Oncol. 2001. PMID: 11740803 Review.
Cited by
- Prevalence and prognostic significance of KIT mutations in pediatric patients with core binding factor AML enrolled on serial pediatric cooperative trials for de novo AML.
Pollard JA, Alonzo TA, Gerbing RB, Ho PA, Zeng R, Ravindranath Y, Dahl G, Lacayo NJ, Becton D, Chang M, Weinstein HJ, Hirsch B, Raimondi SC, Heerema NA, Woods WG, Lange BJ, Hurwitz C, Arceci RJ, Radich JP, Bernstein ID, Heinrich MC, Meshinchi S. Pollard JA, et al. Blood. 2010 Mar 25;115(12):2372-9. doi: 10.1182/blood-2009-09-241075. Epub 2010 Jan 7. Blood. 2010. PMID: 20056794 Free PMC article. - Oncogenic Kit controls neoplastic mast cell growth through a Stat5/PI3-kinase signaling cascade.
Harir N, Boudot C, Friedbichler K, Sonneck K, Kondo R, Martin-Lannerée S, Kenner L, Kerenyi M, Yahiaoui S, Gouilleux-Gruart V, Gondry J, Bénit L, Dusanter-Fourt I, Lassoued K, Valent P, Moriggl R, Gouilleux F. Harir N, et al. Blood. 2008 Sep 15;112(6):2463-73. doi: 10.1182/blood-2007-09-115477. Epub 2008 Jun 25. Blood. 2008. PMID: 18579792 Free PMC article. - Establishment of a FDC-P1 Murine Cell Line with Human KIT N822K Gene Overexpression.
Vagapova ER, Lebedev TD, Popenko VI, Leonova OG, Spirin PV, Prassolov VS. Vagapova ER, et al. Acta Naturae. 2020 Jan-Mar;12(1):51-55. doi: 10.32607/actanaturae.10938. Acta Naturae. 2020. PMID: 32477598 Free PMC article. - Lipid rafts are required for Kit survival and proliferation signals.
Jahn T, Leifheit E, Gooch S, Sindhu S, Weinberg K. Jahn T, et al. Blood. 2007 Sep 15;110(6):1739-47. doi: 10.1182/blood-2006-05-020925. Epub 2007 Jun 6. Blood. 2007. PMID: 17554062 Free PMC article. - Targeted therapy for fusion-driven high-risk acute leukemia.
Pikman Y, Stegmaier K. Pikman Y, et al. Blood. 2018 Sep 20;132(12):1241-1247. doi: 10.1182/blood-2018-04-784157. Epub 2018 Jul 26. Blood. 2018. PMID: 30049809 Free PMC article.
References
- Broudy VC. Stem cell factor and hematopoiesis. Blood. 1997;90: 1345-1364. - PubMed
- Tsujimura T, Furitsu T, Morimoto M, et al. Substitution of an aspartic acid results in constitutive activation of c-kit receptor tyrosine kinase in a rat tumor mast cell line RBL-2H3. Int Arch Allergy Immunol. 1995;106: 377-385. - PubMed
- Pignon JM, Giraudier S, Duquesnoy P, et al. A new c-kit mutation in a case of aggressive mast cell disease. Br J Haematol. 1997;96: 374-376. - PubMed
- Beghini A, Peterlongo P, Ripamonti CB, et al. c-Kit mutations in core binding factor leukemias. Blood. 2000;95: 726-727. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous