The p53 tumor suppressor protein represses human snRNA gene transcription by RNA polymerases II and III independently of sequence-specific DNA binding - PubMed (original) (raw)
The p53 tumor suppressor protein represses human snRNA gene transcription by RNA polymerases II and III independently of sequence-specific DNA binding
Anastasia A Gridasova et al. Mol Cell Biol. 2005 Apr.
Abstract
Human U1 and U6 snRNA genes are transcribed by RNA polymerases II and III, respectively. While the p53 tumor suppressor protein is a general repressor of RNA polymerase III transcription, whether p53 regulates snRNA gene transcription by RNA polymerase II is uncertain. The data presented herein indicate that p53 is an effective repressor of snRNA gene transcription by both polymerases. Both U1 and U6 transcription in vitro is repressed by recombinant p53, and endogenous p53 occupancy at these promoters is stimulated by UV light. In response to UV light, U1 and U6 transcription is strongly repressed. Human U1 genes, but not U6 genes, contain a high-affinity p53 response element located within the core promoter region. Nonetheless, this element is not required for p53 repression and mutant p53 molecules that do not bind DNA can maintain repression, suggesting a reliance on protein interactions for p53 promoter recruitment. Recruitment may be mediated by the general transcription factors TATA-box binding protein and snRNA-activating protein complex, which interact well with p53 and function for both RNA polymerase II and III transcription.
Figures
FIG. 1.
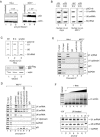
p53 represses human snRNA gene transcription by both RNA polymerases II and III in vitro. (A) Recombinant full-length wild-type p53 and GST proteins were separated by SDS-12.5% PAGE and were stained with Coomassie blue. (B) In vitro transcription from U1, U6, and AdML promoter constructs was tested using HeLa nuclear extracts containing 0, 50, 200, and 800 ng of p53 (lanes 1 to 4) or 800 ng of GST (lane 5). Fifty nanograms of p53 represents an approximate 2:1 molar ratio of monomeric p53 to U1 promoter template DNA and an approximate 8:1 molar ratio to the U6 and AdML promoter plasmids. p53 effectively repressed correctly initiated U1 transcription (U1 5′) and U6 transcription (U6 5′), but it didn't affect read-through (RT) transcription from the U1 reporter plasmid or transcription from the AdML promoter. (C) U1, U6, and 5S rRNA invitro transcription reaction mixtures were supplemented with 800 ng of active or heat-inactivated p53 (lanes 2 to 4 and lanes 5 to 7, respectively) at different times, as indicated. Transcription was allowed to proceed for an additional 60 min. Recombinant p53 repressed U6 gene transcription both prior to and after preinitiation complex assembly but did not repress U1 and 5S rRNA gene transcription after the formation of a preinitiation complex.
FIG. 2.
UV light inhibits snRNA gene transcription and stimulates p53 binding to human snRNA gene promoters. (A) Whole-cell extracts from untreated and UV light-treated HeLa (lanes 1 to 3) and MCF-7 (lanes 4 to 6) cells were analyzed by SDS-12.5% PAGE and Western blot analysis of endogenous p53 and actin. MCF-7 cells exhibited robust accumulation of endogenous p53 in response to UV light treatment, whereas no changewas observed in HeLa cells. (B) UV light represses transcription of endogenous human U1 and U6 snRNA genes in MCF-7 cells, but not in HeLa cells. Nuclear run-on assays measuring polymerase density at U1 snRNA, U6 snRNA, and 5S rRNA genes in nuclei from untreated HeLa cells (lane 1) or MCF-7 cells (lane 3) were compared to results with nuclei harvested 8 h after UV light treatment (lanes 2 and 4). After hybridization, membranes were exposed to film for 7 days. Similar trends were also obtained when exposure times were varied to normalize to GAPDH gene transcription, which was unaffected by UV light treatment in these assays (data not shown). (C) Transiently transfected p53 represses U1 snRNA gene transcription in HeLa cells. Nuclear run-on assays were performed on HeLa cells (lane 1) or HeLa cells transiently transfected with either the empty vector pRC/RSV (lane 2) or pRC-RSV expressing wild-type full-length Flag-tagged p53 (pRc/RSV-p53-Flag) (lane 3). Levels of p53 expression were determined by Western blotting (bottom panel). (D) Endogenous p53 associates with human snRNA gene promoters. Chromatin immunoprecipitation experiments were performed using chromatin harvested from MCF-7 cells prior to or 8 h after UV light treatment and using antibodies directed against SNAP43 (lane 3), various epitopes within p53 (lanes 4 to 7), and nonspecific IgG (lane 8) as a negative control. Enrichment of U1 and U6 promoter regions was measured by PCR and was compared to the p21 promoter (−1.4 kb site), as a positive control, and the U1 upstream region and GAPDH exon 2, as negative controls. (E) Endogenous p53 was not detected at human snRNA gene promoters in untreated or UV light-treated HeLa cells. Chromatin immunoprecipitation experiments were performed using HeLa cell chromatin with the indicated antibodies. (F) UV light causes a decrease in steady-state U1 snRNA levels. (Top panel) Total RNA was isolated from untreated MCF-7 cells and was titrated (0, 0.1, 0.3, 1, 3, and 10 ng [lanes 2 through 7, respectively]) into RT-PCRs performed using U1 gene-specific primers. The amount of U1 cDNA amplification is proportional to the amount of total RNA used for RT-PCR. (Bottom panel) Steady-state levels of U1, U6, and GAPDH RNA were measured by RT-PCR using 2 ng, 10 ng, and 1 μg of total RNA, respectively, harvested before (lane 1) or after (lanes 2 to 7) UV light treatment.
FIG. 3.
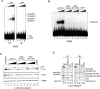
Human U1 snRNA gene core promoters contain a high-affinity p53 binding site. (A) EMSA with increasing amounts of recombinant p53 (0, 80, 160, and 320 ng) were performed with double-stranded DNA probes encompassing various regions of the core U1 promoter, as indicated. At higher p53 concentrations, two p53-dependent complexes were formed on those probes that exhibited high-affinity p53 binding. (B) DNase I footprinting reactions were performed using labeled template stand (lanes 1 to 5) or nontemplate stand (lanes 6 to 10) U1 promoter probes encompassing −150 to +13 with increasing amounts of GST-p53 (0, 100, 200, and 400 ng). Digestion of the probe DNA without added p53 is shown in lanes 1, 5, 6, and 10. The relative positions of the PSE and transcription start site are indicated. Two protected regions within the template strand (labeled F1 and F2) overlap with the three protected regions (labeled F1′, F2′, and F3′) from the nontemplate strand.
FIG.4.
The high-affinity p53 element in the U1 promoter is not essential for p53 repression in vitro. (A) Primary sequence of the U1 core promoter region and the location of the p53 footprints. The plasmid pU1-4.0 contains a wild-type promoter sequence from −151 to +13. Scanning mutagenesis across this region was performed, and the introduced mutations and plasmid identity are indicated. Dots represent positions of identity to this wild-type sequence. (B) Mutations in the p53 footprint 1 region disrupt p53 binding. EMSA was performed using the various U1 promoter probes and either 200 or 400 ng of p53, as indicated (lanes 2 to 37). Lane 1 contains the wild-type U1 promoter probe and no added p53. Mutations within the p53 footprint 1 region caused a marked reduction in p53 binding to the U1 promoter (probes 4.8, 4.9, 4.10, and 4.11). (C) Mutations within the p53 footprint 1 region do not affect p53 repression in vitro. Selected mutations were incorporated into a U1 G-less reporter plasmid for in vitro U1 transcription assays (lanes 1 to 20) in the absence or presence of p53 (800 ng). Lane 21 shows transcription from the wild-type U1 reporter in the presence of GST (800 ng). The read-through (RT) and correctly initiated transcripts (U1 5′) are indicated. (D) p53 represses transcription of the wild-type and mutant U1 reporter constructs to similar extents. (Left panel) An extensive titration of p53 (0, 20, 40, 80, and 160 ng) into U1 transcription assay mixtures was performed using the wild-type (U1 4.0; lanes 1 to 4) and mutant (U1 4.9 + 4.10; lanes 6 to 9) U1 reporter. Lanes 5 and 10 show transcription from the wild-type U1 plasmid in the presence of 160 ng of GST, as a negative control. (Right panel) Transcription levels for two independent experiments were normalized to the signals from transcription reactions containing no added p53, and the average dose-response curves are shown.
FIG. 5.
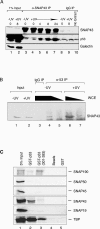
The p53 C terminus is sufficient for transcriptional repression and promoter association. (A) The U6 core promoter does not contain a high-affinity p53 binding element. EMSA was performed with increasing amounts of recombinant p53 (0, 80, 160, and 320 ng) and equivalently labeled double-stranded DNA probes encompassing the U1 core promoter (lanes 1 to 4) and U6 core promoter (lanes 5 to 8). (B) Direct U1 promoter recognition requires the p53 DNA binding domain. EMSA were performed with a wild-type U1 promoter probe (−151 to +13) using 250, 500, and 1,000 ng of full-length wild-type or mutant (R175H) GST-p53 (lanes 2 to 4 and 5 to 7, respectively). Reactions containing truncated GST-p53 (301-393) or GST alone are shown in lanes 8 to 10 and 11 to 13, respectively. Lane 1 contains no added protein. (C) Wild-type and mutant GST-p53 repress transcription similarly. Approximately 100, 200, and 400 ng of wild-type GST-p53 (lanes 2 to 4), GST-p53 (R175H) (lanes 5 to 7), GST-p53 (301-393) (lanes 8 to 10), or 400 ng of GST (lane 11) were titrated into U1, U6, and AdML in vitro transcription reaction mixtures. Both wild-type and mutant p53 molecules repressed U1 and U6 transcription, whereas AdML transcription was unaffected. (D) The p53 DNA binding domain is not required for U1 and U6 promoter association during repression. A portion of untreated or p53 repressed U1 and U6 in vitro transcription reaction mixtures (lanes 1, 4, 7, 10, and 11 in panel C) was cross-linked with formaldehyde and subjected to immunoprecipitation with anti-SNAP43 (lanes 3 and 8), IgG (lanes 4 and 9), or anti-p53 (lanes 5 and 10) antibody. Enrichment of the U1 and U6 reporter plasmids or negative control pUC119 DNA was compared by PCR using promoter-specific or pUC-specific primers. Lanes 1, 2, 6, and 7 show the amplification directly from the input DNA (10 and 1% in lanes 1 and 2 for U1 and lanes 6 and 7 for U6, respectively) contained in the transcription assay mixtures prior to immunoprecipitation. The results of PCRs using the pUC-specific primers under all conditions were indistinguishable and are shown only for the reactions containing added wild-type GST-p53.
FIG. 6.
Endogenous p53 associates with the general transcription factor SNAPC. (A) Immunoprecipitation with anti-SNAP43 antibodies was performed from whole-cell extracts prepared from untreated and UV-treated MCF-7 cells at different time points after UV light treatment. Western analysis revealed significant differences in recovered p53 between the negative control IgG immunoprecipitations (lanes 8 to 10) and anti-SNAP43 immunoprecipitation by the 4-h time point (lane 5). The amount of coimmunoprecipitated p53 continued to increase up to 24 h after UV light treatment (lanes 6 and 7), whereas the amount of precipitated SNAP43 did not change from 0 to 24 h after UV exposure. No galectin-3 was observed in any of the immunopre-cipitations. Lanes 1 and 2 contain 1% of the extract used for immunoprecipitation reactions. (B) Immunoprecipitation with anti-p53 (Ab-6) antibodies was performed from whole-cell extracts (5 mg, lanes 3, 4, and 6; 1.7 mg, lanes 5 and 7) prepared from untreated and UV-treated MCF-7 cells 8 h after UV light treatment, as indicated. Lanes 1 and 2 contain 150 μg of extract that was used as input for immunoprecipitation reactions. Western analysis with anti-SNAP43 antibodies revealed increased SNAP43 association with p53 by 8 h after UV light exposure. (C) p53 interacts with SNAP43, SNAP190, and TBP. GST pull-down experiments were performed with 1 μg of recombinant full-length GST-p53 (lane 2), GST-p53 (301-393) (lane 3), GST (lane 5), or beads alone (lane 4) with individual SNAPC subunits and TBP that were translated in vitro and labeled with [35S]methionine. Lane 1 contains 5% of radiolabeled proteins added to each pull-down reaction mixture.
Similar articles
- Distinct mechanisms for repression of RNA polymerase III transcription by the retinoblastoma tumor suppressor protein.
Hirsch HA, Jawdekar GW, Lee KA, Gu L, Henry RW. Hirsch HA, et al. Mol Cell Biol. 2004 Jul;24(13):5989-99. doi: 10.1128/MCB.24.13.5989-5999.2004. Mol Cell Biol. 2004. PMID: 15199152 Free PMC article. - A variety of RNA polymerases II and III-dependent promoter classes is repressed by factors containing the Krüppel-associated/finger preceding box of zinc finger proteins.
Senatore B, Cafieri A, Di Marino I, Rosati M, Di Nocera PP, Grimaldi G. Senatore B, et al. Gene. 1999 Jul 8;234(2):381-94. doi: 10.1016/s0378-1119(99)00182-1. Gene. 1999. PMID: 10395912 - Regulation of snRNA gene expression by the Drosophila melanogaster small nuclear RNA activating protein complex (DmSNAPc).
Hung KH, Stumph WE. Hung KH, et al. Crit Rev Biochem Mol Biol. 2011 Feb;46(1):11-26. doi: 10.3109/10409238.2010.518136. Epub 2010 Oct 6. Crit Rev Biochem Mol Biol. 2011. PMID: 20925482 Review. - Crossing the line between RNA polymerases: transcription of human snRNA genes by RNA polymerases II and III.
Henry RW, Ford E, Mital R, Mittal V, Hernandez N. Henry RW, et al. Cold Spring Harb Symp Quant Biol. 1998;63:111-20. doi: 10.1101/sqb.1998.63.111. Cold Spring Harb Symp Quant Biol. 1998. PMID: 10384275 Review. No abstract available.
Cited by
- RNA polymerase III transcription in cancer: the BRF2 connection.
Cabarcas S, Schramm L. Cabarcas S, et al. Mol Cancer. 2011 Apr 25;10:47. doi: 10.1186/1476-4598-10-47. Mol Cancer. 2011. PMID: 21518452 Free PMC article. Review. - Transcriptional regulation of human small nuclear RNA genes.
Jawdekar GW, Henry RW. Jawdekar GW, et al. Biochim Biophys Acta. 2008 May;1779(5):295-305. doi: 10.1016/j.bbagrm.2008.04.001. Epub 2008 Apr 8. Biochim Biophys Acta. 2008. PMID: 18442490 Free PMC article. Review. - Differential alterations in metabolic pattern of the spliceosomal UsnRNAs during pre-malignant lung lesions induced by benzo(a)pyrene: modulation by tea polyphenols.
Manna S, Banerjee S, Saha P, Roy A, Das S, Panda CK. Manna S, et al. Mol Cell Biochem. 2006 Sep;289(1-2):149-57. doi: 10.1007/s11010-006-9158-y. Epub 2006 May 23. Mol Cell Biochem. 2006. PMID: 16718374 - Transcriptional regulation by p53.
Beckerman R, Prives C. Beckerman R, et al. Cold Spring Harb Perspect Biol. 2010 Aug;2(8):a000935. doi: 10.1101/cshperspect.a000935. Epub 2010 Apr 28. Cold Spring Harb Perspect Biol. 2010. PMID: 20679336 Free PMC article. Review. - The nucleolus under stress.
Boulon S, Westman BJ, Hutten S, Boisvert FM, Lamond AI. Boulon S, et al. Mol Cell. 2010 Oct 22;40(2):216-27. doi: 10.1016/j.molcel.2010.09.024. Mol Cell. 2010. PMID: 20965417 Free PMC article. Review.
References
- Barlev, N. A., L. Liu, N. H. Chehab, K. Mansfield, K. G. Harris, T. D. Halazonetis, and S. L. Berger. 2001. Acetylation of p53 activates transcription through recruitment of coactivators/histone acetyltransferases. Mol. Cell 8:1243-1254. - PubMed
- Budde, A., and I. Grummt. 1999. p53 represses ribosomal gene transcription. Oncogene 18:1119-1124. - PubMed
- Cain, C., S. Miller, J. Ahn, and C. Prives. 2000. The N terminus of p53 regulates its dissociation from DNA. J. Biol. Chem. 275:39944-39953. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous