Krüppel-like factor 4 prevents centrosome amplification following gamma-irradiation-induced DNA damage - PubMed (original) (raw)
Krüppel-like factor 4 prevents centrosome amplification following gamma-irradiation-induced DNA damage
Hong S Yoon et al. Oncogene. 2005.
Abstract
Centrosome duplication is a carefully controlled process in the cell cycle. Previous studies indicate that the tumor suppressor, p53, regulates centrosome duplication. Here, we present evidence for the involvement of the mammalian Krüppel-like transcription factor, KLF4, in preventing centrosome amplification following DNA damage caused by gamma-irradiation. The colon cancer cell line HCT116, which contains wild-type p53 alleles (HCT116 p53+/+), displayed stable centrosome numbers following gamma-irradiation. In contrast, HCT116 cells null for the p53 alleles (HCT116 p53-/-) exhibited centrosome amplification after irradiation. In the latter cell line, KLF4 was not activated following gamma-irradiation due to the absence of p53. However, centrosome amplification could be suppressed in irradiated HCT116 p53-/- cells by conditional induction of exogenous KLF4. Conversely, in a HCT116 p53+/+ cell line stably transfected with small hairpin RNA (shRNA) designed to specifically inhibit KLF4, gamma-irradiation induced centrosome amplification. In these cells, the inability of KLF4 to become activated in response to DNA damage was directly associated with an increase in cyclin E level and Cdk2 activity, both essential for regulating centrosome duplication. Cotransfection experiments showed that KLF4 overexpression suppressed the promoter activity of the cyclin E gene. The results of this study demonstrated that KLF4 is both necessary and sufficient in preventing centrosome amplification following gamma-radiation-induced DNA damage and does so by transcriptionally suppressing cyclin E expression.
Figures
Figure 1
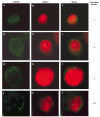
Centrosome immunostaining in HCT116 _p53_−/− cells following γ-irradiation. HCT116 _p53_−/− cells were irradiated with 12 Gy γ-ray and stained for centrosomes using a γ-tubulin antibody 2 days later. Examples of a cell with 1, 2, 3, or >4 centrosomes are shown. Nucleus was counterstained with Topro3
Figure 2
Centrosome profiles of HCT116 p53+/+ and _p53_−/− cells with and without γ-irradiation and of irradiated EcR116 _p53_−/− cells infected with AdEGI-KLF4. HCT116 p53+/+ (a) and _p53_−/− (b) cells were irradiated (+γ) or not (−γ) on day 0 and maintained in culture for 2 days before stained for centrosomes. A total of 200 cells with visible centrosomes were scored in each experiment and the experiments were conducted three additional times for a total N of 4. *P<0.001. (c) EcR116 _p53_−/− cells were infected with recombinant adenovirus containing KLF4, AdEGI-KLF4 (Chen et al., 2001), and irradiated with 12 Gy of γ-ray. Cells were then treated (+PA) or not (−PA) with ponasterone A (PA) to induce KLF4 expression. Centrosomes were scored 2 days later. A total of 200 cells were counted in each experiment and the experiments were repeated three additional times for a total N of 4. *P<0.05
Figure 3
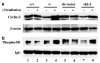
Establishment of stable cell lines with reduced KLF4 expression by RNA interference. HCT116 p53+/+ cells were stably transfected with a plasmid containing small hairpin RNA (shRNA) directed against KLF4 or vector alone. Two independent clones were derived and named KLF4/sh2-1 and KLF4/sh2-2. Cells were irradiated or not on day 0 and maintained in culture for 2 days before being harvested for Western blot analysis for p53, KLF4, p21WAF1/CIP1, and β-actin. Sh-vector indicates cells transfected with vector alone
Figure 4
Mitotic indices and centrosome profiles of HCT116 KLF4/sh2-2 cells with and without irradiation. HCT116 KLF4/sh2-2 cells were irradiated (+γ) or not (−γ) (a) on day 0 and maintained in culture for 2 days before being examined for the presence of mitotic figures. N = 4. *P<0.01. In (b), the cells were irradiated (+γ) or not (−γ) on day 0 and maintained in culture for 2 days before staining for centrosomes. A total of 300 cells were examined in each experiment and the experiments were repeated two additional times for a total N of 3. *P<0.01
Figure 5
Western blot analysis of cyclin E and Cdk2 kinase activity in irradiated and unirradiated cells. HCT116 p53+/+ (lanes 1 and 2), _p53_−/− (lanes 3 and 4), HCT116 p53+/+ transfected with sh-vector (lanes 5 and 6) and HCT116 p53+/+ transfected with KLF4/sh2-2 vector (lanes 7 and 8) were irradiated with 12 Gy (+), or not (−). After 2 days, extracts were prepared for Western blot analysis of cyclin E and β-actin (a) or Cdk2 kinase assay (b). Phospho-H1 is detected with a phospho-specific antibody against histone H1. Immunoglobulin G (IgG) is used as a loading control
Figure 6
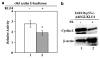
KLF4 suppresses cyclin E expression. (a)HCT116 _p53_−/− cells were cotransfected with the −363 cyclin E-luciferase reporter, Renilla luciferase internal control, and PMT3 (lane 1) or PMT3-KLF4 (lane 2). Luciferase activity was determined 2 days following transfection and normalized to the internal control. The means of six independent experiments are shown. *P<0.005. (b)EcR116 _p53_−/− cells were infected with AdEGI-KLF4, irradiated with 12 Gy, and treated (lane 2) or not (lane 1) with the inducer, ponasterone A (PA). Cell extracts were prepared 2 days later and examined for the content of cyclin E and β-actin by Western blotting
Similar articles
- Requirement of Krüppel-like factor 4 in preventing entry into mitosis following DNA damage.
Yoon HS, Yang VW. Yoon HS, et al. J Biol Chem. 2004 Feb 6;279(6):5035-41. doi: 10.1074/jbc.M307631200. Epub 2003 Nov 19. J Biol Chem. 2004. PMID: 14627709 Free PMC article. - Kruppel-like factor 4 mediates p53-dependent G1/S cell cycle arrest in response to DNA damage.
Yoon HS, Chen X, Yang VW. Yoon HS, et al. J Biol Chem. 2003 Jan 24;278(4):2101-5. doi: 10.1074/jbc.M211027200. Epub 2002 Nov 8. J Biol Chem. 2003. PMID: 12427745 Free PMC article. - Suppression of centrosome amplification after DNA damage depends on p27 accumulation.
Sugihara E, Kanai M, Saito S, Nitta T, Toyoshima H, Nakayama K, Nakayama KI, Fukasawa K, Schwab M, Saya H, Miwa M. Sugihara E, et al. Cancer Res. 2006 Apr 15;66(8):4020-9. doi: 10.1158/0008-5472.CAN-05-3250. Cancer Res. 2006. PMID: 16618721 - Loss of p53 and centrosome hyperamplification.
Tarapore P, Fukasawa K. Tarapore P, et al. Oncogene. 2002 Sep 9;21(40):6234-40. doi: 10.1038/sj.onc.1205707. Oncogene. 2002. PMID: 12214254 Review. - Roles of BRCA1 in centrosome duplication.
Deng CX. Deng CX. Oncogene. 2002 Sep 9;21(40):6222-7. doi: 10.1038/sj.onc.1205713. Oncogene. 2002. PMID: 12214252 Review.
Cited by
- Tough beginnings: alterations in the transcriptome of cloned embryos during the first two cell cycles.
Vassena R, Han Z, Gao S, Baldwin DA, Schultz RM, Latham KE. Vassena R, et al. Dev Biol. 2007 Apr 1;304(1):75-89. doi: 10.1016/j.ydbio.2006.12.015. Epub 2006 Dec 12. Dev Biol. 2007. PMID: 17234177 Free PMC article. - Aging and the Krüppel-like factors.
Hsieh PN, Sweet DR, Fan L, Jain MK. Hsieh PN, et al. Trends Cell Mol Biol. 2017;12:1-15. Trends Cell Mol Biol. 2017. PMID: 29416266 Free PMC article. - New insight into the significance of KLF4 PARylation in genome stability, carcinogenesis, and therapy.
Zhou Z, Huang F, Shrivastava I, Zhu R, Luo A, Hottiger M, Bahar I, Liu Z, Cristofanilli M, Wan Y. Zhou Z, et al. EMBO Mol Med. 2020 Dec 7;12(12):e12391. doi: 10.15252/emmm.202012391. Epub 2020 Nov 24. EMBO Mol Med. 2020. PMID: 33231937 Free PMC article. - The role of Krüppel-like factors in the reprogramming of somatic cells to induced pluripotent stem cells.
Nandan MO, Yang VW. Nandan MO, et al. Histol Histopathol. 2009 Oct;24(10):1343-55. doi: 10.14670/HH-24.1343. Histol Histopathol. 2009. PMID: 19688699 Free PMC article. Review. - Transcriptional profiling of the cell cycle checkpoint gene krüppel-like factor 4 reveals a global inhibitory function in macromolecular biosynthesis.
Whitney EM, Ghaleb AM, Chen X, Yang VW. Whitney EM, et al. Gene Expr. 2006;13(2):85-96. doi: 10.3727/000000006783991908. Gene Expr. 2006. PMID: 17017123 Free PMC article.
References
- Al-Romaih K, Bayani J, Vorobyova J, Karaskova J, Park PC, Zielenska M, Squire JA. Cancer Genet. Cytogenet. 2003;144:91–99. - PubMed
- Bennett RA, Izumi H, Fukasawa K. Oncogene. 2004;23:6823–6829. - PubMed
- Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Science. 1998;282:1497–1501. - PubMed
- Bunz F, Fauth C, Speicher MR, Dutriaux A, Sedivy JM, Kinzler KW, Vogelstein B, Lengauer C. Cancer Res. 2002;62:1129–1133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK052230/DK/NIDDK NIH HHS/United States
- T32 GM008169/GM/NIGMS NIH HHS/United States
- DK64399/DK/NIDDK NIH HHS/United States
- R01 CA084197/CA/NCI NIH HHS/United States
- R24 DK064399/DK/NIDDK NIH HHS/United States
- DK52230/DK/NIDDK NIH HHS/United States
- CA84197/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous