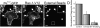Phosphatidylinositol-4,5-bisphosphate hydrolysis directs actin remodeling during phagocytosis - PubMed (original) (raw)
Phosphatidylinositol-4,5-bisphosphate hydrolysis directs actin remodeling during phagocytosis
Cameron C Scott et al. J Cell Biol. 2005.
Abstract
The Rho GTPases play a critical role in initiating actin polymerization during phagocytosis. In contrast, the factors directing the disassembly of F-actin required for fission of the phagocytic vacuole are ill defined. We used fluorescent chimeric proteins to monitor the dynamics of association of actin and active Cdc42 and Rac1 with the forming phagosome. Although actin was found to disappear from the base of the forming phagosome before sealing was complete, Rac1/Cdc42 activity persisted, suggesting that termination of GTPase activity is not the main determinant of actin disassembly. Furthermore, fully internalized phagosomes engineered to associate constitutively with active Rac1 showed little associated F-actin. The disappearance of phosphatidylinositol-4,5-bisphosphate (PI(4,5)P(2)) from the phagosomal membrane closely paralleled the course of actin disassembly. Furthermore, inhibition of PI(4,5)P(2) hydrolysis or increased PI(4,5)P(2) generation by overexpression of phosphatidylinositol phosphate kinase I prevented the actin disassembly necessary for the completion of phagocytosis. These observations suggest that hydrolysis of PI(4,5)P(2) dictates the remodeling of actin necessary for completion of phagocytosis.
Figures
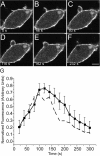
Figure 1.
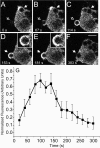
Association of GFP-actin with forming phagosomes. Phagocytosis was initiated by addition of IgG-opsonized latex beads (3.1-μm diam) to RAW cells stably transfected with GFP-actin. Fluorescence was monitored by confocal microscopy. (A–F) Representative time course. The numbers indicate the time in seconds after a bead makes contact at the site indicated by the open arrow in A. Note that another bead has just been engulfed near the top of the cell and still shows remnant actin at the site of sealing. Insets show enlargements of the phagosome noted by arrow. Open arrows point to forming phagosomes and closed arrows point to formed, sealed phagosomes. Bar, 5 μm. (G) The phagosomal accumulation of GFP-actin above the cytosolic level was quantified and binned into 20-s intervals as detailed in Materials and methods. Abscissa: time in seconds after the bead made contact with cell. Ordinate: relative fluorescence. To allow comparison between experiments, phagosomal fluorescence was normalized to the maximum recorded for each individual phagosome. Data are means ± SEM of seven individual determinations.
Figure 2.
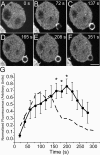
Assessment of Rac/Cdc42 activity in forming phagosomes. Phagocytosis was initiated by addition of IgG-opsonized latex beads to RAW cells transiently transfected with PBD-YFP. Fluorescence was monitored by confocal microscopy. (A–F) Representative time course. The numbers indicate the time in seconds after a bead makes contact with the cell. Bar, 5 μm. (G) The phagosomal accumulation of PBD-YFP above the cytosolic level was quantified, normalized and binned into 20-s intervals as in Fig. 1 (solid line). Abscissa: time in seconds after the bead made contact with cell. Ordinate: relative fluorescence, normalized as in Fig. 1 G. Data are means ± SEM of six individual determinations. The dashed line is a reproduction of the GFP-actin data of Fig. 1, for comparison. Asterisks indicate instances where the difference between the curves is significant (P < 0.05).
Figure 3.
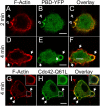
Comparison of Rac/Cdc42 activity and F-actin in forming phagosomes. RAW cells were transiently transfected with the PBD-YFP chimeric probe (B, C, E, and F, green) or with Cdc42Q61L-GFP (H and I, green) and exposed to IgG-opsonized latex beads for the time indicated. The cells were immediately fixed with 4% PFA, permeabilized and stained with rhodamine-phalloidin to visualize F-actin (A, C, D, F, G, and I, red). (A–C) PBD-YFP expressing cells fixed 2 min after addition of beads. (D–F) PBD-YFP expressing cells fixed 4 min after addition of beads. (G–I) Cdc42Q61L-GFP expressing cells fixed after 4 min. Open arrows point to forming phagosomes and closed arrows point to sealed phagosomes. Inset in G–I shows enlargement of phagosome denoted by the box in main panel. Bars, 5 μm. Images are representative of at least three experiments of each type.
Figure 4.
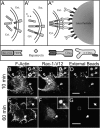
**Actin association with myc-Rac1-V12–induced phagosomes. (**A) Strategy used to generate phagosomes by recruitment of active Rac1 to the membrane. RBL-2H3 cells were stably transfected with two separate constructs: a soluble, myc-tagged Rac1-V12/FKBP2 chimera (myc-Rac1-V12) and a transmembrane CD25/FRB chimera (A). Association between the two chimeras is induced by addition of rapamycin (A′). Beads coated with anti-CD25 antibodies are then added to cross-link the complexes at defined sites (A′′). (B–D) After binding the beads, the cells were incubated for 10 min at 37°C, then rapidly cooled to 4°C and treated with Cy5-conjugated anti–mouse antibodies to identify beads that were accessible from the medium, i.e., not completely internalized. The cells were next fixed, permeabilized, and stained for F-actin and immunostained with anti-myc antibodies to reveal the location of myc-Rac1-V12. (E–G) After binding the beads, the cells were incubated for 60 min at 37°C and treated as in B–D. Bars, 5 μm. Open arrows point to forming phagosomes and closed arrows point to sealed phagosomes. Insets show a magnification of the area indicated by the square in the main panels. Images are representative of three experiments.
Figure 5.
Association of PI(4,5)P2 with forming phagosomes. Phagocytosis was initiated by addition of IgG-opsonized latex beads to RAW cells transiently transfected with PHPLCδ-GFP. Fluorescence was monitored by confocal microscopy. (A–F) Representative time course. The numbers indicate the time in seconds after a bead makes contact with the cell. Bar, 5 μm. (G) The phagosomal accumulation of PHPLCδ-GFP above the cytosolic level was quantified, normalized, and binned into 20-s intervals as in Fig. 1 (solid line). Data are means ± SEM of eight individual determinations. The dashed line is a reproduction of the GFP-actin data of Fig. 1, for comparison. The curves were not significantly different (P > 0.05) at any point.
Figure 6.
Disappearance of PI(4,5)P2 from Rac1-V1–induced phagosomes. RBL-2H3 cells engineered to express stably the two constructs described in Fig. 4 were transiently transfected with PHPLCδ-GFP (A). Beads coated with anti-CD25 antibodies were then added and the cells were incubated for 60 min at 37°C, then rapidly cooled to 4°C and treated with Cy5-conjugated anti–mouse antibodies to identify beads that were accessible from the medium, i.e., not completely internalized (C). A small amount of light refracted by latex is visible in both internal and external beads. This refraction is apparent only at the center of the beads and is clearly distinguishable from the more peripheral antibody labeling. The cells were next fixed, permeabilized and immunostained with anti-myc antibodies to reveal the location of myc-Rac1-V12 (B). Open arrows point to externally accessible beads and closed arrows point to sealed phagosomes. Bar, 5 μm. Insets show a magnification of the area indicated by the square in the main panels. (D) Phagocytosis was induced in the transfected RBL-2H3 cells as described in Fig. 4. The cells were otherwise untreated, or were pretreated with the PLC inhibitors U73122 (1 μM) or ET-18-OCH3 (25 μM) for 10 min before phagocytosis. Extracellular beads were labeled and the number of beads ingested after 20 min was quantified under a fluorescence microscope. The phagocytic index was normalized to allow comparison of multiple experiments. The data are the means ± SEM of three experiments, each scoring at least 50 cells.
Figure 7.
Effect of PLC inhibition on F-actin distribution. IgG-opsonized latex beads were added to RAW cells that were otherwise untreated (A–C) or that had been pretreated with 1 μM U73122 for 10 min (D–F). After 15 min at 37°C, the cells were cooled to 4°C and extracellular beads identified by addition of FITC-conjugated secondary antibody (B and E). The cells were next fixed, permeabilized, and stained with rhodamine-phalloidin (A and D). Corresponding differential interference contrast (DIC) images are shown in C and F. Insets show a magnification of the area indicated by the square in the main panels. Open arrows point to externally accessible beads and closed arrows point to sealed phagosomes. (G) Cortical F-actin thickness, quantified from line scans (white bars) and total cortical actin, calculated by integration (black bars) in control and in U73122-treated cells. Data are the means ± SEM of three experiments, each scoring at least 20 cells. (H) High throughput analysis of F-actin during phagocytosis, determined by averaging the integrated fluorescence of rhodamine-phalloidin in populations of cells. Circles, moderate number of beads. Triangles, high number of beads. Stars, moderate number of beads added after pretreatment with 5 μM U73122 for 10 min. Data show the integrated fluorescence of at least 2,500 cells per time point. Standard error bars were smaller than the size of the symbols.
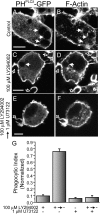
Figure 8.
Effect of PLC inhibition on the completion of phagocytosis. RAW cells transiently transfected with PHPLCδ-GFP were either left untreated (A and B), treated with 100 μM LY294002 for 30 min (C and D), treated with 1 μM U73122 for 10 min (not depicted), or treated with 100 μM LY294002 for 30 min, with 1 μM U73122 present for the last 10 min of the incubation, followed by removal of LY294002 while maintaining the U73122 for an additional 10 min (E and F). The cells were then exposed to IgG-opsonized particles for 10 min and finally fixed, permeabilized, and stained with rhodamine-phalloidin (B, D, and F). PHPLCδ-GFP fluorescence is shown in A, C, and E. Bars, 5 μm. (G) The phagocytic index of cells treated as above was determined as described in Materials and methods. Data are the means ± SEM of three experiments, each scoring at least 50 cells.
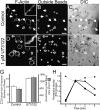
Figure 9.
Effect of PIPKI overexpression on phagocytosis. RAW cells transfected with the plasmids indicated below were exposed to IgG-opsonized particles for 8–10 min, then analyzed by laser confocal microscopy either immediately (A and B) or after fixation and staining with Alexa 633-phalloidin (D) or rhodamine-phalloidin (E and F). (A and B) RAW cells transiently transfected with PIPKIβ-YFP and PHPLCδ-CFP. (C and D) Cells transiently transfected with PIPKIβ-YFP. (E and F) Cells transiently transfected with PIPKIγ-GFP. (G) Quantification of the phagocytic efficiency of untransfected cells (control) or cells transiently transfected with PIPKIα-CFP, PIPKIβ-YFP, or PIPKIγ-GFP. Data are the means ± SEM of three experiments, each scoring at least 100 cells.
Similar articles
- Cdc42, Rac1, and Rac2 display distinct patterns of activation during phagocytosis.
Hoppe AD, Swanson JA. Hoppe AD, et al. Mol Biol Cell. 2004 Aug;15(8):3509-19. doi: 10.1091/mbc.e03-11-0847. Epub 2004 May 28. Mol Biol Cell. 2004. PMID: 15169870 Free PMC article. - A Cdc42 activation cycle coordinated by PI 3-kinase during Fc receptor-mediated phagocytosis.
Beemiller P, Zhang Y, Mohan S, Levinsohn E, Gaeta I, Hoppe AD, Swanson JA. Beemiller P, et al. Mol Biol Cell. 2010 Feb 1;21(3):470-80. doi: 10.1091/mbc.e08-05-0494. Epub 2009 Dec 2. Mol Biol Cell. 2010. PMID: 19955216 Free PMC article. - ArhGAP12 plays dual roles in Stabilin-2 mediated efferocytosis: Regulates Rac1 basal activity and spatiotemporally turns off the Rac1 to orchestrate phagosome maturation.
Bae DJ, Seo J, Kim SY, Park SY, Do Yoo J, Pyo JH, Cho W, Cho JY, Kim S, Kim IS. Bae DJ, et al. Biochim Biophys Acta Mol Cell Res. 2019 Oct;1866(10):1595-1607. doi: 10.1016/j.bbamcr.2019.07.003. Epub 2019 Jul 10. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 31301364 - Regulation of the actin cytoskeleton by PIP2 in cytokinesis.
Logan MR, Mandato CA. Logan MR, et al. Biol Cell. 2006 Jun;98(6):377-88. doi: 10.1042/BC20050081. Biol Cell. 2006. PMID: 16704377 Review. - Modular components of phagocytosis.
Greenberg S. Greenberg S. J Leukoc Biol. 1999 Nov;66(5):712-7. doi: 10.1002/jlb.66.5.712. J Leukoc Biol. 1999. PMID: 10577498 Review.
Cited by
- PtdIns(4,5)P₂ and PtdIns3P coordinate to regulate phagosomal sealing for apoptotic cell clearance.
Cheng S, Wang K, Zou W, Miao R, Huang Y, Wang H, Wang X. Cheng S, et al. J Cell Biol. 2015 Aug 3;210(3):485-502. doi: 10.1083/jcb.201501038. J Cell Biol. 2015. PMID: 26240185 Free PMC article. - Regulation of phosphatidylinositol 3-kinase by polyisoprenyl phosphates in neutrophil-mediated tissue injury.
Bonnans C, Fukunaga K, Keledjian R, Petasis NA, Levy BD. Bonnans C, et al. J Exp Med. 2006 Apr 17;203(4):857-63. doi: 10.1084/jem.20052143. Epub 2006 Mar 27. J Exp Med. 2006. PMID: 16567384 Free PMC article. - Quantitative analysis of membrane remodeling at the phagocytic cup.
Lee WL, Mason D, Schreiber AD, Grinstein S. Lee WL, et al. Mol Biol Cell. 2007 Aug;18(8):2883-92. doi: 10.1091/mbc.e06-05-0450. Epub 2007 May 16. Mol Biol Cell. 2007. PMID: 17507658 Free PMC article. - An electrostatic switch displaces phosphatidylinositol phosphate kinases from the membrane during phagocytosis.
Fairn GD, Ogata K, Botelho RJ, Stahl PD, Anderson RA, De Camilli P, Meyer T, Wodak S, Grinstein S. Fairn GD, et al. J Cell Biol. 2009 Nov 30;187(5):701-14. doi: 10.1083/jcb.200909025. J Cell Biol. 2009. PMID: 19951917 Free PMC article. - Stochastic geometry sensing and polarization in a lipid kinase-phosphatase competitive reaction.
Hansen SD, Huang WYC, Lee YK, Bieling P, Christensen SM, Groves JT. Hansen SD, et al. Proc Natl Acad Sci U S A. 2019 Jul 23;116(30):15013-15022. doi: 10.1073/pnas.1901744116. Epub 2019 Jul 5. Proc Natl Acad Sci U S A. 2019. PMID: 31278151 Free PMC article.
References
- Aderem, A. 2002. How to eat something bigger than your head. Cell. 110:5–8. - PubMed
- Aderem, A., and D.M. Underhill. 1999. Mechanisms of phagocytosis in macrophages. Annu. Rev. Immunol. 17:593–623. - PubMed
- Allison, A.C., P. Davies, and S. De Petris. 1971. Role of contractile microfilaments in macrophage movement and endocytosis. Nat. New Biol. 232:153–155. - PubMed
- Anes, E., M.P. Kuhnel, E. Bos, J. Moniz-Pereira, A. Habermann, and G. Griffiths. 2003. Selected lipids activate phagosome actin assembly and maturation resulting in killing of pathogenic mycobacteria. Nat. Cell Biol. 5:793–802. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous