ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow - PubMed (original) (raw)
ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow
Lin Yang et al. Blood. 2005.
Abstract
In vivo, leukocyte transendothelial migration (TEM) occurs at endothelial cell junctions (paracellular) and nonjunctional (transcellular) locations, whereas in vitro models report that TEM is mostly paracellular. The mechanisms that control the route of leukocyte TEM remain unknown. Here we tested the hypothesis that elevated intercellular adhesion molecule-1 (ICAM-1) expression regulates the location of polymorphonuclear leukocyte (PMN) TEM. We used an in vitro flow model of tumor necrosis factor-alpha (TNF-alpha)-activated human umbilical vein endothelium cells (HUVECs) or an HUVEC cell line transfected with ICAM-1GFP (green fluorescent protein) and live-cell fluorescence microscopy to quantify the location of PMN adhesion and TEM. We observed robust transcellular TEM with TNF-alpha-activated HUVECs and ICAM-1GFP immortalized HUVECS (iHUVECs). In contrast, primary CD3+ T lymphocytes exclusively used a paracellular route. Endothelial ICAM-1 was identified as essential for both paracellular and transcellular PMN transmigration, and interfering with ICAM-1 cytoplasmic tail function preferentially reduced transcellular TEM. We also found that ICAM-1 surface density and distribution as well as endothelial cell shape contributed to transcellular TEM. In summary, ICAM-1 promotes junctional and nonjunctional TEM across inflamed vascular endothelium via distinct cytoplasmic tail associations.
Figures
Figure 1.
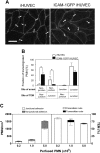
HUVECs expressing high levels of ICAM-1 support PMN transcellular TEM. (A) Fluorescent micrographs of live HUVEC monolayers treated with media containing TNF-α for 4 hours or 24 hours or media alone (0 hours), as indicated, and then stained with the nonfunction-blocking ICAM-1 mAb CL23.4 conjugated with Alexa 488. The images were captured using identical settings and were processed identically as detailed in “Materials and methods” to create the images shown. Bar, 20 μm, × 40 objective, 0.75 NA. (B) The surface expression of ICAM-1 on 4-hour (purple line) or 24-hour (red line) TNF-α-activated HUVECs was assessed by indirect immunofluorescence staining and flow cytometry as previously described. Dashed line indicates nonbinding mAb; solid black line, media alone (0 hours after TNF-α treatment). (C) Confluent primary HUVECs or ICAM-1GFP iHUVECs were treated with TNF-α for 4 hours or 24 hours, as indicated. Endothelial cell monolayers were stained with Alexa 568-conjugated anti-VE-cadherin mAb (Alexa568/hec-1) to identify cell-cell lateral junctions. The aspect ratio (length to width) was determined by measuring the maximal length and width of the cell outlined by VE-cadherin staining. *P ≤ .05. (D) PMNs (5 × 105/mL) in flow buffer (DPBS plus 0.1% HSA) were drawn across control, 4- or 24-hour TNF-α-activated HUVEC monolayers that had been stained with fluorescently labeled VE-cadherin mAb as described in “Materials and methods.” PMN accumulation and location of TEM were determined as follows: sequential DIC and corresponding VE-cadherin red fluorescence images were overlaid in register to create a time-lapse movie using MetaMorph software. The VE-cadherin fluorescence identified the endothelial cell-cell junctions. Neutrophils that arrested or transmigrated at locations greater than 5 μm from cell-cell junctions, as identified by VE-cadherin staining, were considered nonjunctional events. n = 6 from 3 independent experiments. *P < .05. The black and gray error bars correspond to paracellular and transcellular route, respectively. All data are mean ± SD. (E) Sequential images recorded by live-cell imaging under high power (× 40 objective), depict a PMN (arrow) that arrested and then transmigrated at a nonjunctional site on 24-hour TNF-α-activated HUVECs. No change in VE-cadherin staining was observed during TEM of this PMN. Bar, 10 μm.
Figure 2.
Characterization of ICAM-1GFP iHUVECs. (A) Surface expression of ICAM-1 on 4-hour TNF-α-activated ICAM-1GFP iHUVECs (red lines) was assessed by indirect immunofluorescence staining and flow cytometry and compared with that of 4-hour (left) and 24-hour TNF-α-activated iHUVECs (right; black solid line). Dotted line indicates nonbinding mAb. (B) iHUVECs or ICAM-1GFP iHUVECs were treated with TNF-α for 4 hours or 24 hours or media alone (0 hours), as indicated. Expression of endogenous ICAM-1 and ICAM-1GFP was determined by immunoprecipitation of biotinylated ICAM-1 on cell surface, as described in “Materials and methods.” (C) Surface distribution pattern of ICAM-1 on 4-hour TNF-α-activated iHUVECs and ICAM-1GFP iHUVECs was detected by live-cell epifluorescence microscopy (× 40 objective; top) and confocal microscopy (× 60 objective; bottom) as described in “Materials and methods.” Orthogonal views of endothelium were reproduced from serial z-sections horizontally scanned using confocal microscopy (0.5-μm increments) and manipulated with Lasersharp 2000 V4.3. To visualize ICAM-1, live iHUVECs were stimulated with TNF-α for 4 hours and incubated with Alexa 488-labeled anti-ICAM-1 mAb CL23.4, washed, and observed by confocal microscopy. ICAM-1GFP on 4-hour TNF-α-activated ICAM-1GFP iHUVECs was detected by GFP signal (not Alexa 488 anti-ICAM-1 mAb). (D) Live confluent HUVECs, iHUVECs, and ICAM-1GFP iHUVECs were stained with Alexa 568-labeled anti-JAM-A mAb 1H2A9 or anti-VE-cadherin TEA1/31. Images were obtained by the live-cell epifluorescence imaging as detailed in “Materials and methods.”
Figure 3.
Enhanced ICAM-1 expression in HUVECs directs transcellular TEM. (A) PMN accumulation and TEM on 4-hour TNF-α-activated iHUVECs, GFP iHUVECs, or ICAM-1GFP iHUVECs were assessed as described in “Materials and methods” and Figure 1D legend. (B) Increasing numbers of PMNs (as indicated) were perfused across HUVEC monolayers under lower shear stress (1 dyne/cm2). Accumulation of PMNs (left) and the route of TEM (right) were determined. (C) PMNs (106/mL) were perfused across HUVEC monolayers at the estimated shear stress indicated. The route of TEM (right) was determined as in “Materials and methods.” n = 3 experiments. (D) Electron microscopy of PMNs undergoing transcellular TEM of 4-hour TNF-α-activated ICAM-1GFP iHUVECs under static conditions. In the top panel, 2 PMNs have extended granule-containing and, in one case, nuclear-lobe-containing cell processes, completely through the endothelial cell thickness from their apical to basilar surfaces. These endothelial cell holes are bound by endothelial cell plasma membranes and are located fewer then 5 μm (the cutoff used to group migrating cells in the “junctional route” category) from adjacent closed junctions (J1 and J2) and apposed lateral borders (arrows) of individual endothelial cells in the HUVEC monolayer. In lower panel, another PMN is nearly completely beneath the HUVEC layer after passing through an endothelial cell hole fewer than 5 μm from the adjacent closed junction (arrow) between 2 endothelial cells (EC1 and EC2). An organelle-free, actin-rich, cellular process (arrowhead) still spans the endothelial cell hole. Magnification top, × 18 000; lower, × 20 500. Bar, 1 μm. (E) Live-cell imaging of ICAM-1 GFP iHUVEC monolayers was performed as detailed to monitor redistribution of ICAM-1 during PMN transcellular TEM under shear flow (1 dyne/cm2). Arrows identify 2 PMNs that undergo transcellular TEM (see Video S2). Bars, 20 μm. *P < .05.
Figure 4.
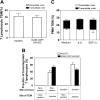
The location of PMN adhesion and subsequent transmigration on ICAM-1GFP iHUVEC monolayers. (A) The initial adhesion sites of PMNs and the sites of TEM were determined from review of individual images using ImageJ, and these positions were overlaid onto the VE-cadherin staining pattern graph obtained prior to introduction of PMNs into the chamber. Bar, 20 μm. (B) The transmigrated PMNs were grouped according to their sites of initial arrest and TEM at junction or nonjunction (> 5 μm from any junction). The fraction of PMNs in each group was calculated. *P < .05. The black and gray error bars correspond to paracellular and transcellular routes, respectively. (C) Increasing numbers of PMNs (as indicated) were drawn across 4-hour TNF-α iHUVEC monolayers at 0.5 dyne/cm2. Initial location of arrested PMNs (left) and the route of TEM (right) were determined as detailed in “Materials and methods.”
Figure 5.
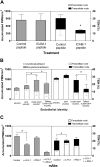
Transcellular TEM requires intact ICAM-1. (A) ICAM-1GFP iHUVECs were incubated with penatratin-ICAM-1 peptide or control penetratin peptide (100 μg/mL, 2 hours). PMN accumulation and the route of TEM were assessed at 1 dyne/cm2 as described in Figure 1D legend. (B) Initial location of PMN adhesion and TEM at 1 dyne/cm2 were assessed for iHUVECs, GFP iHUVECs, ICAM-1GFP iHUVECs, and tailless ICAM-1GFP iHUVECs. (C) PMN accumulation and TEM were assessed for the 4-hour TNF-α-stimulated endothelial monolayers after preincubation of PMNs with function-blocking mAb to LFA-1 (TS 1/22) or Mac-1 (mAb 44), or both. A second mAb to Mac-1 (LPM19c, data not shown) gave similar results. *P < .05.
Figure 6.
Elevated ICAM-1 expression does not trigger T-lymphocyte transcellular TEM. The site of T-lymphocyte arrest and subsequent TEM, or PMN location of TEM across 4-hour TNF-α-activated iHUVECs or ICAM-1 GFP iHUVECs was determined by live-cell imaging as in the legend of Figure 4. (A) The percentage of T lymphocytes that transmigrated by paracellular or transcellular routes was determined. (B) The fraction of transmigrated T lymphocytes with different sites of arrest and TEM of ICAM-1GFP iHUVECs, grouped as in Figure 4A, were determined. (C) Four-hour TNF-α-activated ICAM-1GFP iHUVECs were incubated for 15 minutes with 100 ng/mL recombinant human IL-8, 10 μg/mL SDF-1α, or buffer alone and then assayed for PMN TEM as detailed in the legend of Figure 4.
Similar articles
- NO reduces PMN adhesion to human vascular endothelial cells due to downregulation of ICAM-1 mRNA and surface expression.
Lindemann S, Sharafi M, Spiecker M, Buerke M, Fisch A, Grosser T, Veit K, Gierer C, Ibe W, Meyer J, Darius H. Lindemann S, et al. Thromb Res. 2000 Feb 1;97(3):113-23. doi: 10.1016/s0049-3848(99)00162-0. Thromb Res. 2000. PMID: 10680642 - Role of ICAM-1 and ICAM-2 and alternate CD11/CD18 ligands in neutrophil transendothelial migration.
Issekutz AC, Rowter D, Springer TA. Issekutz AC, et al. J Leukoc Biol. 1999 Jan;65(1):117-26. doi: 10.1002/jlb.65.1.117. J Leukoc Biol. 1999. PMID: 9886254 - ICAM-1-mediated, Src- and Pyk2-dependent vascular endothelial cadherin tyrosine phosphorylation is required for leukocyte transendothelial migration.
Allingham MJ, van Buul JD, Burridge K. Allingham MJ, et al. J Immunol. 2007 Sep 15;179(6):4053-64. doi: 10.4049/jimmunol.179.6.4053. J Immunol. 2007. PMID: 17785844 - Hug tightly and say goodbye: role of endothelial ICAM-1 in leukocyte transmigration.
Rahman A, Fazal F. Rahman A, et al. Antioxid Redox Signal. 2009 Apr;11(4):823-39. doi: 10.1089/ars.2008.2204. Antioxid Redox Signal. 2009. PMID: 18808323 Free PMC article. Review. - Endothelial signaling in paracellular and transcellular leukocyte transmigration.
Wittchen ES. Wittchen ES. Front Biosci (Landmark Ed). 2009 Jan 1;14(7):2522-45. doi: 10.2741/3395. Front Biosci (Landmark Ed). 2009. PMID: 19273217 Free PMC article. Review.
Cited by
- Endothelial cell, myeloid, and adaptive immune responses in SARS-CoV-2 infection.
Degauque N, Haziot A, Brouard S, Mooney N. Degauque N, et al. FASEB J. 2021 May;35(5):e21577. doi: 10.1096/fj.202100024R. FASEB J. 2021. PMID: 33831263 Free PMC article. Review. - A novel phosphocholine-mimetic inhibits a pro-inflammatory conformational change in C-reactive protein.
Zeller J, Cheung Tung Shing KS, Nero TL, McFadyen JD, Krippner G, Bogner B, Kreuzaler S, Kiefer J, Horner VK, Braig D, Danish H, Baratchi S, Fricke M, Wang X, Kather MG, Kammerer B, Woollard KJ, Sharma P, Morton CJ, Pietersz G, Parker MW, Peter K, Eisenhardt SU. Zeller J, et al. EMBO Mol Med. 2023 Jan 11;15(1):e16236. doi: 10.15252/emmm.202216236. Epub 2022 Dec 5. EMBO Mol Med. 2023. PMID: 36468184 Free PMC article. - Modulation of endothelial cell integrity and inflammatory activation by commercial lipid emulsions.
Harvey KA, Xu Z, Pavlina TM, Zaloga GP, Siddiqui RA. Harvey KA, et al. Lipids Health Dis. 2015 Feb 18;14:9. doi: 10.1186/s12944-015-0005-6. Lipids Health Dis. 2015. PMID: 25888960 Free PMC article. - A systematic review and meta-analysis of circulating adhesion molecules in rheumatoid arthritis.
Mangoni AA, Zinellu A. Mangoni AA, et al. Inflamm Res. 2024 Mar;73(3):305-327. doi: 10.1007/s00011-023-01837-6. Epub 2024 Jan 19. Inflamm Res. 2024. PMID: 38240792 Free PMC article. Review. - Simvastatin Reduces Neutrophils Infiltration Into Brain Parenchyma After Intracerebral Hemorrhage via Regulating Peripheral Neutrophils Apoptosis.
Zhang J, Shi X, Hao N, Chen Z, Wei L, Tan L, Chen Y, Feng H, Chen Q, Zhu G. Zhang J, et al. Front Neurosci. 2018 Dec 18;12:977. doi: 10.3389/fnins.2018.00977. eCollection 2018. Front Neurosci. 2018. PMID: 30631264 Free PMC article.
References
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76: 301-314. - PubMed
- Cinamon G, Shinder V, Alon R. Wall shear forces promote lymphocyte migration across inflamed vascular endothelium presenting apical chemokines. Nat Immunol. 2001;2: 515-522. - PubMed
- Muller WA. Migration of leukocytes across endothelial junctions: some concepts and controversies. Microcirculation. 2001;8: 181-193. - PubMed
- Kvietys PR, Sandig M. Neutrophil diapedesis: paracellular or transcellular? News Physiol Sci. 2001;16: 15-19. - PubMed
- Engelhardt B, Wolburg H. Mini-review: transendothelial migration of leukocytes: through the front door or around the side of the house? Eur J Immunol. 2004;34: 2955-2963. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL36028/HL/NHLBI NIH HHS/United States
- R01 HL053993/HL/NHLBI NIH HHS/United States
- P01 HL036028/HL/NHLBI NIH HHS/United States
- AI33372/AI/NIAID NIH HHS/United States
- HL56985/HL/NHLBI NIH HHS/United States
- HL53993/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous