Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of mu- and delta- but not kappa-opioids - PubMed (original) (raw)
Comparative Study
Spinal G-protein-gated potassium channels contribute in a dose-dependent manner to the analgesic effect of mu- and delta- but not kappa-opioids
Cheryl L Marker et al. J Neurosci. 2005.
Abstract
Opioids can evoke analgesia by inhibiting neuronal targets in either the brain or spinal cord, and multiple presynaptic and postsynaptic inhibitory mechanisms have been implicated. The relative significance of presynaptic and postsynaptic inhibition to opioid analgesia is essentially unknown, as are the identities and relevant locations of effectors mediating opioid actions. Here, we examined the distribution of G-protein-gated potassium (GIRK) channels in the mouse spinal cord and measured their contribution to the analgesia evoked by spinal administration of opioid receptor-selective agonists. We found that the GIRK channel subunits GIRK1 and GIRK2 were concentrated in the outer layer of the substantia gelatinosa of the dorsal horn. GIRK1 and GIRK2 were found almost exclusively in postsynaptic membranes of putative excitatory synapses, and a significant degree of overlap with the mu-opioid receptor was observed. Although most GIRK subunit labeling was perisynaptic or extrasynaptic, GIRK2 was found occasionally within the synaptic specialization. Genetic ablation or pharmacologic inhibition of spinal GIRK channels selectively blunted the analgesic effect of high but not lower doses of the mu-opioid receptor-selective agonist [D-Ala(2),N-Me-Phe(4),Gly(5)-ol]-enkephalin. Dose-dependent contributions of GIRK channels to the analgesic effects of the -opioid receptor-selective agonists Tyr-D-Ala-Phe-Glu-Val-Val-Gly amide and [D-Pen(2,5)]-enkephalin were also observed. In contrast, the analgesic effect of the agonist (trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidinyl)-cyclohexyl] benzeneacetamide methanesulfonate hydrate was preserved despite the absence of GIRK channels. We conclude that the activation of postsynaptic GIRK1 and/or GIRK2-containing channels in the spinal cord dorsal horn represents a powerful, albeit relatively insensitive, means by which intrathecal mu- and -selective opioid agonists evoke analgesia.
Figures
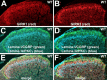
Figure 1.
Laminar expression of GIRK subunits in the spinal cord dorsal horn. A, B, Expression of GIRK1 (A) and GIRK2 (B) in the dorsal horn of the spinal cord, shown in serial sections (10 μm) taken from the lumbar enlargement of a wild-type (WT) mouse. Note the concentrated staining for both GIRK1 and GIRK2 in the superficial layer of the dorsal horn. Sections from wild-type mice were processed on the same slide with sections from GIRK1 knock-out and GIRK2 knock-out mice to verify the specificity of GIRK antibody staining (data not shown). The images are representative of data from three different mice. C, D, The same sections were costained with antibodies for the lamina I marker CGRP (green) and PKCγ (blue), a marker for the inner aspect of lamina II (Lamina IIi). E, F, Overlays of the GIRK subunit, CGRP, and PKCγ staining patterns in each section. The region of most concentrated staining for GIRK1 and GIRK2 was found in lamina IIo.
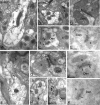
Figure 2.
Subcellular distribution of GIRK channels in the spinal cord dorsal horn. A-K, Electron micrographs showing the subcellular distribution of GIRK1 (A-E) and GIRK2 (F-K) subunits in the dorsal horn (lamina IIo) of the mouse spinal cord. Den, Dendritic shafts; b, axon bouton. A-C, Using a preembedding immunogold technique, GIRK1 immunoreactivity was observed along the extrasynaptic plasma membrane (arrows) of dendritic shafts, postsynaptic to excitatory axon terminals. D, GIRK1 immunoparticles were also observed at the edge of asymmetrical synapses (crossed arrow), postsynaptic to excitatory axon terminals. E, Using a postembedding immunogold technique, GIRK1 immunoreactivity was detected at perisynaptic (crossed arrow) and extrasynaptic (arrow) locations but never within the postsynaptic specialization. F-H, Using the preembedding immunogold technique, GIRK2 immunoreactivity was localized to the extrasynaptic plasma membrane (arrows) of dendritic shafts establishing asymmetrical synapses with axon boutons and was associated with asymmetrical synapses (e.g., crossed arrow in G). I, To a much lesser extent, GIRK2 immunoreactivity was observed in the terminal portion (arrowheads) of axon boutons establishing asymmetrical synapses with dendritic shafts. J, K, Using the postembedding immunogold method, GIRK2 immunoparticles were found within the synaptic specialization of asymmetrical synapses (double arrowheads). Scale bars: A, 0.5 μm; B-K, 0.2 μm.
Figure 3.
Analgesia evoked by MOR agonists in the absence of GIRK channels or MORs. The analgesic effect of intrathecal DAMGO and morphine was measured using the tail-flick test. Comparable data were obtained at three different bath temperatures (49, 52.5, and 55°C), and, as such, only the 52.5°C data are presented here. A, The analgesic effect (%MPE) of intrathecal morphine (0, 0.3, and 3 nmol) was measured in wild-type (black bars; n = 18 per dose) and MOR knock-out (KO; gray bars; n = 5-6 per dose) mice. *p < 0.05 versus MOR KO (same dose); +p < 0.05 versus 0 nmol (same genotype); ++p < 0.01 versus 0 and 0.3 nmol (same genotype). B, The analgesic effect (%nMPE) of intrathecal DAMGO (0, 10, 30, 100, and 200 pmol) was measured in wild-type male C57BL/6 mice in the absence (control; white circles) or presence (black circles) of 30 pmol of tertiapin. Group sizes ranged from 10 to 16 mice per drug condition. *p < 0.05 versus tertiapin-treated mice (same dose). C, The analgesic effect (%MPE) of intrathecal DAMGO (0, 30, and 200 pmol) was measured in wild-type (white bars; n = 19-21 mice per dose), GIRK1 knock-out (black bars; n = 13-14 mice per dose), and GIRK2 knock-out (gray bars; n = 11-13 mice per dose) mice. *p < 0.05 versus GIRK knock-out mice (same dose). Error bars represent SEM.
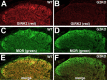
Figure 4.
Overlap between GIRK subunits and MORs in the dorsal horn. Sections (10 μm) from the lumbar enlargement of wild-type (WT; A, C, E) and GIRK2 knock-out (G2KO; B, D, F) mice were stained with antibodies directed against GIRK2 (A, B; red) and MORs (C, D; green), with the overlays presented in E and F. The data presented are representative of staining patterns observed in sections from three different mice. Comparable images were obtained with GIRK1 antibodies as well with tissue from GIRK1 knock-out mice (data not shown).
Figure 5.
Subcellular distribution of MORs and GIRK channels in the dorsal horn. Electron micrographs showing MOR and GIRK subunit labeling in the dorsal horn (lamina IIo). Den, Dendritic shaft; b, axon bouton. A-D, MOR immunoparticles were found in the extrasynaptic (arrows) and perisynaptic plasma membrane (crossed arrows) of dendritic shafts establishing asymmetrical synapses with presynaptic boutons. MOR immunoreactivity was also observed in axon boutons establishing asymmetrical synapses with dendritic shafts, either along the extrasynaptic plasma membrane (arrowhead) or within active zones (double arrowheads). E, F, Postsynaptic colocalization of MORs and GIRK2 using a preembedding immunogold method. The HRP reaction product represents MOR immunoreactivity, and it filled dendritic shafts with GIRK2 immunoparticles organized along the extrasynaptic plasma membrane (arrows). G, Using a postembedding immunogold method, GIRK2 immunoparticles (10 nm) and MOR immunoparticles (20 nm) were found postsynaptically within the same asymmetrical synapse. H, Presynaptic colocalization of MORs and GIRK2 using a preembedding immunogold method. The HRP reaction product (MOR immunoreactivity) filled the axon terminal, whereas GIRK2 immunoparticles were found along the extrasynaptic plasma membrane (arrowhead). Note the postsynaptic GIRK2 immunoparticle (arrow). I, J, Postsynaptic colocalization of MORs and GIRK1 using a preembedding immunogold method. The HRP reaction product (MOR immunoreactivity) filled dendritic shafts, whereas GIRK1 immunoparticles were found along the extrasynaptic plasma membrane (arrows). Scale bars, 0.2 μm.
Figure 6.
Analgesia evoked by DOR agonists in the absence of GIRK channels. A, B, The analgesic effects of intrathecal DPDPE (0, 1, 3, 10, and 30 nmol) or deltorphin II (0, 0.3, 1, 3, and 10 pmol) were measured with the tail-flick test in wild-type male C57BL/6 mice in the absence (control; white circles) or presence (black circles) of 30 pmol of tertiapin. Comparable data were obtained at three different bath temperatures (49, 52.5, and 55°C), and, as such, only the 52.5°C data are presented here. Group sizes ranged from 10 to 16 mice per drug condition. *p < 0.05 versus tertiapin-treated mice (same dose). C, The analgesic effect of intrathecal deltorphin II (0, 30, and 200 pmol) was measured in wild-type (white bars; n = 11-17 per dose), GIRK1 knock-out (KO; black bars; n = 8-9 per dose), and GIRK2 knock-out (gray bars; n = 5-8 per dose) mice. *p < 0.05 versus GIRK knock-out mice. Error bars represent SEM.
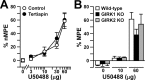
Figure 7.
Analgesia evoked by a KOR agonist in the absence of GIRK channels. The analgesic effect of intrathecal U50488 was measured using the tail-flick test. Comparable data were obtained at three different bath temperatures (49, 52.5, and 55°C), and, as such, only the 52.5°C data are presented here. A, The analgesic effect of intrathecal U50488 (0, 3, 10, 30, and 60 μg) was measured in wild-type male C57BL/6 mice in the absence (saline; white circles) or presence (tertiapin; black circles) of 30 pmol of tertiapin. Group sizes ranged from 9 to 16 mice per dose. B, The analgesic effect of intrathecal U50488 (0, 10, and 60 μg) was measured in wild-type (white bars; n = 15 per dose), GIRK1 knock-out (KO; black bars; n = 6-13 per dose), and GIRK2 knock-out (gray bars; n = 5-7 per dose) mice. Error bars represent SEM.
Similar articles
- Distinct populations of spinal cord lamina II interneurons expressing G-protein-gated potassium channels.
Marker CL, Luján R, Colón J, Wickman K. Marker CL, et al. J Neurosci. 2006 Nov 22;26(47):12251-9. doi: 10.1523/JNEUROSCI.3693-06.2006. J Neurosci. 2006. PMID: 17122050 Free PMC article. - Mu Receptors.
Herman TF, Cascella M, Muzio MR. Herman TF, et al. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31855381 Free Books & Documents. - G-protein-gated Inwardly Rectifying Potassium Channels Modulate Respiratory Depression by Opioids.
Montandon G, Ren J, Victoria NC, Liu H, Wickman K, Greer JJ, Horner RL. Montandon G, et al. Anesthesiology. 2016 Mar;124(3):641-50. doi: 10.1097/ALN.0000000000000984. Anesthesiology. 2016. PMID: 26675532 Free PMC article. - Molecular mechanisms of analgesia induced by opioids and ethanol: is the GIRK channel one of the keys?
Ikeda K, Kobayashi T, Kumanishi T, Yano R, Sora I, Niki H. Ikeda K, et al. Neurosci Res. 2002 Oct;44(2):121-131. doi: 10.1016/s0168-0102(02)00094-9. Neurosci Res. 2002. PMID: 12354627 Review. - Opioidergic control of the spinal release of neuropeptides. Possible significance for the analgesic effects of opioids.
Bourgoin S, Benoliel JJ, Collin E, Mauborgne A, Pohl M, Hamon M, Cesselin F. Bourgoin S, et al. Fundam Clin Pharmacol. 1994;8(4):307-21. doi: 10.1111/j.1472-8206.1994.tb00809.x. Fundam Clin Pharmacol. 1994. PMID: 7851837 Review.
Cited by
- New insights into the therapeutic potential of Girk channels.
Luján R, Marron Fernandez de Velasco E, Aguado C, Wickman K. Luján R, et al. Trends Neurosci. 2014 Jan;37(1):20-9. doi: 10.1016/j.tins.2013.10.006. Epub 2013 Nov 21. Trends Neurosci. 2014. PMID: 24268819 Free PMC article. Review. - Participation of K(ATP) Channels in the Antinociceptive Effect of Pregabalin in Rat Formalin Test.
Kweon TD, Kim JY, Kwon IW, Choi JB, Lee YW. Kweon TD, et al. Korean J Pain. 2011 Sep;24(3):131-6. doi: 10.3344/kjp.2011.24.3.131. Epub 2011 Sep 6. Korean J Pain. 2011. PMID: 21935490 Free PMC article. - Inactivation of GIRK channels weakens the pre- and postsynaptic inhibitory activity in dorsal raphe neurons.
Llamosas N, Ugedo L, Torrecilla M. Llamosas N, et al. Physiol Rep. 2017 Feb;5(3):e13141. doi: 10.14814/phy2.13141. Physiol Rep. 2017. PMID: 28196855 Free PMC article. - Group III metabotropic glutamate receptors: guardians against excitotoxicity in ischemic brain injury, with implications for neonatal contexts.
Mielecki D, Salińska E. Mielecki D, et al. Pharmacol Rep. 2024 Dec;76(6):1199-1218. doi: 10.1007/s43440-024-00651-z. Epub 2024 Sep 17. Pharmacol Rep. 2024. PMID: 39298028 Free PMC article. Review. - High-Intensity Swimming Exercise Decreases Glutamate-Induced Nociception by Activation of G-Protein-Coupled Receptors Inhibiting Phosphorylated Protein Kinase A.
Martins DF, Siteneski A, Ludtke DD, Dal-Secco D, Santos ARS. Martins DF, et al. Mol Neurobiol. 2017 Sep;54(7):5620-5631. doi: 10.1007/s12035-016-0095-9. Epub 2016 Sep 13. Mol Neurobiol. 2017. PMID: 27624384
References
- Atweh S, Kuhar M (1977) Autoradiographic localization of opiate receptors in rat brain I. Spinal cord and lower medulla. Brain Res 124: 53-67. - PubMed
- Beckstead MJ, Grandy DK, Wickman K, Williams JT (2004) Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron 42: 939-946. - PubMed
- Besse D, Lombard M, Zajac J, Roques B, Besson J (1990) Pre- and postsynaptic distribution of mu, delta, and kappa opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Res 521: 15-22. - PubMed
- Bettahi I, Marker CL, Roman MI, Wickman K (2002) Contribution of the Kir3.1 subunit to the muscarinic-gated atrial potassium channel IKACh J Biol Chem 277: 48282-48288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DA00564/DA/NIDA NIH HHS/United States
- R01 DA000564/DA/NIDA NIH HHS/United States
- R01 MH061933/MH/NIMH NIH HHS/United States
- DA01583/DA/NIDA NIH HHS/United States
- R01 DA001583/DA/NIDA NIH HHS/United States
- MH61933/MH/NIMH NIH HHS/United States
- T32 DA07234/DA/NIDA NIH HHS/United States
- P50 DA011806/DA/NIDA NIH HHS/United States
- T32 DA007234/DA/NIDA NIH HHS/United States
- R56 DA000564/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials