Activated STAT5 proteins induce activation of the PI 3-kinase/Akt and Ras/MAPK pathways via the Gab2 scaffolding adapter - PubMed (original) (raw)
Activated STAT5 proteins induce activation of the PI 3-kinase/Akt and Ras/MAPK pathways via the Gab2 scaffolding adapter
Rémy Nyga et al. Biochem J. 2005.
Abstract
The active forms of STAT5A (signal transducer and activator of transcription 5A) and STAT5B are able to relieve the cytokine dependence of haematopoietic cells and to induce leukaemia in mice. We have demonstrated previously that activation of the PI3K (phosphoinositide 3-kinase) signalling cascade plays a major role in cell growth and survival induced by these proteins. Interaction between STAT5 and p85, the regulatory subunit of the PI3K, has been suggested to be required for this activation. We show in the present study that the scaffolding protein Gab2 [Grb2 (growth-factor-receptor-bound protein 2)-associated binder-2] is an essential component of this interaction. Gab2 is persistently tyrosine-phosphorylated in Ba/F3 cells expressing caSTAT5 (constitutively activated STAT5), independent of JAK2 (Janus kinase 2) activation where it interacts with STAT5, p85 and Grb2, but not with Shp2 [SH2 (Src homology 2)-domain-containing tyrosine phosphatase] proteins. Interaction of STAT5 with Gab2 was also observed in Ba/F3 cells stimulated with interleukin-3 or expressing the oncogenic fusion protein Tel-JAK2. The MAPKs (mitogen-activated protein kinases) ERK1 (extracellular-signal-regulated kinase 1) and ERK2 were constitutively activated in the caSTAT5-expressing cells and were found to be required for caSTAT5-induced cell proliferation. Overexpression of Gab2-3YF, a mutant of Gab2 incapable of binding PI3K, inhibited the proliferation and survival of caSTAT5-expressing cells as well as ERK1/2 and Akt/protein kinase B phosphorylation. Taken together, our results indicate that Gab2 is required for caSTAT5-induced cell proliferation by regulating both the PI3K/Akt and the Ras/MAPK pathways.
Figures
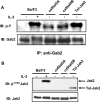
Figure 1. Interaction between STAT5, Gab2 and p85
(A) Schematic representation of the _ca_STAT5 mutants (STAT5A1*6 and STAT5B1*6). The regulatory domains and mutations are shown. (B) Cell lysates were prepared from Ba/F3 cells stimulated (lane 2) or not (lane 1) with IL-3 (10 ng/ml) for 30 min or from Ba/F3 cells expressing _ca_STAT5A (lane 3), _ca_STAT5B (lane 4) or Tel–JAK2 (lane 5). The p85 subunit of PI3K was immunoprecipitated with specific antibodies and the presence of co-precipitated STAT5, IRS-2 and Gab2 was specifically assessed by Western blotting. The membrane was reprobed with anti-p85 antibodies. (C) The same cell lysates were used for immunoprecipitation with anti-STAT5 (left panel) and anti-Gab2 (right panel), then analysed by Western blotting for the presence of p85 and Gab2 or anti-p85 and STAT5 respectively. The membranes were reprobed with the antibodies used in immunoprecipitation. (D) STAT5 was immunoprecipitated from _ca_STAT5-containing cell lysates that have been or not immunodepleted of Gab2. The association of p85 with STAT5 was next determined by immunobloting with anti-p85 antibodies.
Figure 2. Gab2 is tyrosine-phosphorylated in _ca_STAT5-expressing Ba/F3 cells
(A) NP40 cell lysates were first subjected to immunoprecipitation with an anti-Gab2 antibody and then analysed by immunoblotting with an anti-phosphotyrosine antibody (4G10). The membrane was reprobed with anti-Gab2 antibody. (B) Total cell lysates were subjected to SDS/PAGE and then immunoblotted with anti-phospho-Tyr1007-JAK2 antibody. The membrane was reprobed with an anti-JAK2 antibody.
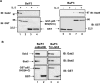
Figure 3. In vitro association of Gab2, STAT5 and the SH2 domains of p85
(A) Cell lysates were prepared from Ba/F3 cells stimulated or not with IL-3 for 30 min and incubated with glutathione–agarose beads coupled with either GST protein alone (lanes 3–6) or GST-p85-SH2 (N+C) (lanes 1, 2, 7 and 8). Proteins eluted from the beads were analysed by Western blotting with anti-Gab2 (left panel) or anti-STAT5 (right panel) antibodies. Membranes were reprobed with anti-GST antibody. (B) A similar experiment was conducted with cell extracts from _ca_STAT5B- and Tel–JAK2-expressing cells. Interaction of STAT5 and Gab2 with GST-p85-SH2 (N+C) (lanes 1 and 3) or GST alone (lanes 2 and 4) was determined by immunoblotting. The membrane was reprobed with the anti-GST antibody.
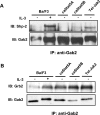
Figure 4. Gab2 associates with Grb2 but not with Shp2 in _ca_STAT5-expressing Ba/F3 cells
Anti-Gab2 immunoprecipitates obtained from NP40 cell lysates were analysed by immunoblotting for the presence of Shp2 (A) or Grb2 (B). Membranes were reprobed with the anti-Gab2 antibody.
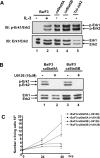
Figure 5. Constitutive activation of ERK1 and ERK2 in _ca_STAT5-expressing Ba/F3 cells: role in proliferation
(A) Total cell lysates from Ba/F3 cells unstimulated (lane 1) or stimulated (lane 2) with IL-3 (10 ng/ml for 30 min) or from _ca_STAT5A- (lane 3), _ca_STAT5B- (lane 4) and Tel–JAK2- (lane 5) expressing cells were subjected to SDS/PAGE and immunoblotted with anti-phospho-ERK1/ERK2 antibody. The membrane was reprobed with an anti-ERK1/ERK2 antibody. (B) _ca_STAT5A- and _ca_STAT5B-expressing Ba/F3 cells were left untreated (ethanol) (lanes 1 and 3) or treated with U0126 (10 μM) for 24 h (lanes 2 and 4). Total cell lysates were prepared and analysed by Western blotting with anti-phospho-ERK1/ERK2 antibody. The membrane was reprobed with an anti-ERK1/ERK2 antibody. (C) _ca_STAT5A- and _ca_STAT5B-expressing Ba/F3 cells were treated or not with U0126 (10 μM) for the indicated time periods and the number of viable cells was determined daily, using the Trypan Blue dye exclusion method. Results shown are representative of three experiments.
Figure 6. Overexpression of Gab2-3YF inhibits growth and induces apoptosis of the Ba/F3 cells expressing _ca_STAT5
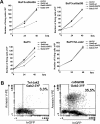
(A) Cells were electroporated with the different Gab2 constructs or the empty vector as indicated. On the following day, GFP+ cells were sorted by flow cytometry and analysed for growth by cell counting using the Trypan Blue dye exclusion method. Results shown are representative of three different transfection assays. (B) _ca_STAT5B- and Tel–JAK2-expressing Ba/F3 cells were labelled with annexin V–PE 24 h post-transfection, and the percentage of double positive (annexin V+, GFP+) apoptotic cells was determined by flow cytometry.
Figure 7. Overexpression of Gab2-3YF inhibits Akt and ERK1/ERK2 phosphorylation in _ca_STAT5-expressing Ba/F3 cells
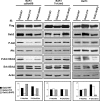
Ba/F3 cells (right panel) and Tel–JAK2- (middle panel) and _ca_STAT5B- (left panel) expressing cells were electroporated with the FLAG-tagged Gab2 constructs as indicated. GFP+ cells were sorted 24 h later and the content of transfected Gab2 was determined by immunoblotting with anti-FLAG or anti-Gab2 antibodies. The levels of phosphorylated Akt and ERK1/2 in the different transfected cells were also determined by immunoblotting with the indicated antibodies and quantified by densitometric analysis. The ratio of phosphorylated versus total Akt and ERK1/ERK2 proteins is expressed in arbitrary units (bottom panels).
Similar articles
- Constitutively active STAT5 variants induce growth and survival of hematopoietic cells through a PI 3-kinase/Akt dependent pathway.
Santos SC, Lacronique V, Bouchaert I, Monni R, Bernard O, Gisselbrecht S, Gouilleux F. Santos SC, et al. Oncogene. 2001 Apr 19;20(17):2080-90. doi: 10.1038/sj.onc.1204308. Oncogene. 2001. PMID: 11360192 - Cooperation between STAT5 and phosphatidylinositol 3-kinase in the IL-3-dependent survival of a bone marrow derived cell line.
Rosa Santos SC, Dumon S, Mayeux P, Gisselbrecht S, Gouilleux F. Rosa Santos SC, et al. Oncogene. 2000 Feb 24;19(9):1164-72. doi: 10.1038/sj.onc.1203418. Oncogene. 2000. PMID: 10713704 - TEL-Syk fusion constitutively activates PI3-K/Akt, MAPK and JAK2-independent STAT5 signal pathways.
Kanie T, Abe A, Matsuda T, Kuno Y, Towatari M, Yamamoto T, Saito H, Emi N, Naoe T. Kanie T, et al. Leukemia. 2004 Mar;18(3):548-55. doi: 10.1038/sj.leu.2403266. Leukemia. 2004. PMID: 14749700 - Structure and function of Gab2 and its role in cancer (Review).
Ding CB, Yu WN, Feng JH, Luo JM. Ding CB, et al. Mol Med Rep. 2015 Sep;12(3):4007-4014. doi: 10.3892/mmr.2015.3951. Epub 2015 Jun 17. Mol Med Rep. 2015. PMID: 26095858 Free PMC article. Review. - Targets of B-cell antigen receptor signaling: the phosphatidylinositol 3-kinase/Akt/glycogen synthase kinase-3 signaling pathway and the Rap1 GTPase.
Gold MR, Ingham RJ, McLeod SJ, Christian SL, Scheid MP, Duronio V, Santos L, Matsuuchi L. Gold MR, et al. Immunol Rev. 2000 Aug;176:47-68. doi: 10.1034/j.1600-065x.2000.00601.x. Immunol Rev. 2000. PMID: 11043767 Review.
Cited by
- Interleukin-2 Receptor β Thr-450 Phosphorylation Is a Positive Regulator for Receptor Complex Stability and Activation of Signaling Molecules.
Ruiz-Medina BE, Ross JA, Kirken RA. Ruiz-Medina BE, et al. J Biol Chem. 2015 Aug 21;290(34):20972-20983. doi: 10.1074/jbc.M115.660654. Epub 2015 Jul 7. J Biol Chem. 2015. PMID: 26152718 Free PMC article. - Quantitative phosphoproteomic analysis reveals unique cAMP signaling pools emanating from AC2 and AC6 in human airway smooth muscle cells.
Cattani-Cavalieri I, Li Y, Margolis J, Bogard A, Roosan MR, Ostrom RS. Cattani-Cavalieri I, et al. Front Physiol. 2023 Feb 28;14:1149063. doi: 10.3389/fphys.2023.1149063. eCollection 2023. Front Physiol. 2023. PMID: 36926196 Free PMC article. - GRB2-associated binding protein 2 regulates multiple pathways associated with the development of prostate cancer.
Qiao XR, Zhang X, Mu L, Tian J, Du Y. Qiao XR, et al. Oncol Lett. 2020 Oct;20(4):99. doi: 10.3892/ol.2020.11960. Epub 2020 Aug 7. Oncol Lett. 2020. PMID: 32831918 Free PMC article. - Function, regulation and pathological roles of the Gab/DOS docking proteins.
Wöhrle FU, Daly RJ, Brummer T. Wöhrle FU, et al. Cell Commun Signal. 2009 Sep 8;7:22. doi: 10.1186/1478-811X-7-22. Cell Commun Signal. 2009. PMID: 19737390 Free PMC article. - The Impact of ETV6-NTRK3 Oncogenic Gene Fusions on Molecular and Signaling Pathway Alterations.
Kinnunen M, Liu X, Niemelä E, Öhman T, Gawriyski L, Salokas K, Keskitalo S, Varjosalo M. Kinnunen M, et al. Cancers (Basel). 2023 Aug 24;15(17):4246. doi: 10.3390/cancers15174246. Cancers (Basel). 2023. PMID: 37686522 Free PMC article.
References
- Kisseleva T., Bhattacharya S., Braunstein J., Schindler C. W. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24. - PubMed
- Buitenhuis M., Coffer P. J., Koenderman L. Signal transducer and activator of transcription 5 (Stat5) Int. J. Biochem. Cell Biol. 2004;36:2120–2124. - PubMed
- Moriggl R., Topham D. J., Teglund S., Sexl V., McKay C., Wang D., Hoffmeyer A., van Deursen J., Sangster M. Y., Bunting K. D., et al. Stat5 is required for IL-2-induced cell cycle progression of peripheral T cells. Immunity. 1999;10:249–259. - PubMed
- Sexl V., Piekorz R., Moriggl R., Rohrer J., Brown M. P., Bunting K. D., Rothammer K., Roussel M. F., Ihle J. N. Stat5a/b contribute to interleukin 7-induced B-cell precursor expansion, but abl- and bcr/abl-induced transformation are independent of stat5. Blood. 2000;96:2277–2283. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous