Isolation of neural stem cells from the postnatal cerebellum - PubMed (original) (raw)
Isolation of neural stem cells from the postnatal cerebellum
Audra Lee et al. Nat Neurosci. 2005 Jun.
Abstract
The cerebellum is critical for motor coordination and cognitive function and is the target of transformation in medulloblastoma, the most common malignant brain tumor in children. Although the development of granule cells, the most abundant neurons in the cerebellum, has been studied in detail, the origins of other cerebellar neurons and glia remain poorly understood. Here we show that the murine postnatal cerebellum contains multipotent neural stem cells (NSCs). These cells can be prospectively isolated based on their expression of the NSC marker prominin-1 (CD133) and their lack of markers of neuronal and glial lineages (lin-). Purified prominin+ lin- cells form self-renewing neurospheres and can differentiate into astrocytes, oligodendrocytes and neurons in vitro. Moreover, they can generate each of these lineages after transplantation into the cerebellum. Identification of cerebellar stem cells has important implications for the understanding of cerebellar development and the origins of medulloblastoma.
Figures
Figure 1
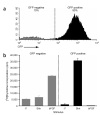
Non-granule cell precursors can be purified from the postnatal cerebellum. (a) Isolation of non-GCPs. Cells from neonatal Math1-GFP cerebellum were sorted into GFP-negative and GFP-positive populations by flow cytometry. (b) Proliferative responses of non-GCPs. The GFP- and GFP+ populations were cultured with no stimulus (ø), Shh or bFGF and then harvested to measure thymidine incorporation. Data represent triplicates ± s.e.m.
Figure 2
Prominin+ cells proliferate in response to bFGF. GFP- cells from Math1-GFP cerebella were sorted by FACS into prominin+ and prominin- cells, and each population was cultured either with no stimulus (Ø) or with bFGF and then harvested for analysis of thymidine incorporation. Data represent triplicates ± s.e.m.
Figure 3
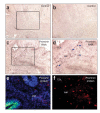
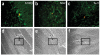
Prominin+ cells are located in the cerebellar white matter. (a-d) Detection of prominin mRNA by in situ hybridization. Cryosections of P7 cerebellum were hybridized with digoxigenin (DIG)-labeled sense (control; a,b) or antisense (c,d) probes specific for mouse Prom1 (prominin RNA) and then stained with alkaline phosphatase-conjugated antibodies to DIG and with NBT/BCIP substrate to detect bound probe. Sections were photographed at 10× (a,c)and 20× (b,d) magnification; boxes indicate locations of regions shown in b,d. Arrows indicate prominin+ cells (dark brown spots) in the white matter (WM). (e,f) Detection of prominin protein by immunostaining. cerebellar sections were stained with rat antibodies to prominin-1 and TRITC-conjugated secondary antibodies. Low-power (10)image of cerebellum from a Math1-GFP mouse shows GFP fluorescence× (green) in the outer EGL and prominin+ cells (red) in the white matter (e). The section is counterstained with DAPI (blue) to highlight cerebellar structure. High-power (20×) image of cerebellum from wild-type mouse shows prominin+ cells (red) in the white matter (f). No counterstain is shown. Red, green and blue images were photographed separately and merged with Openlab software. EGL, external germinal layer; WM, white matter.
Figure 4
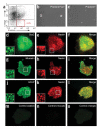
Prominin+lin- cells generate neurospheres. (a) Isolation of prominin+lin- cells. cerebellar cells from Math1-GFP mice were stained with antibodies to prominin-1 (x axis) and neuronal and glial lineage markers (PSA-NCAM, TAPA-1 and O4, y axis), and analyzed by flow cytometry. Prominin+lin- cells represent 0.2% of the total population isolated from the postnatal cerebellum. (b,c) Neurosphere formation. FACS-sorted prominin+lin- and prominin- cells were cultured at clonal density in the presence of bFGF and EGF for 10 d. (c-o) Expression of NSC markers. Neurospheres from clonal cultures of prominin+lin- cells were stained with rabbit antibodies specific for Sox-2 (d), Musashi (g)or GFAP (j) and with mouse antibodies specific for nestin (e,h,k); primary antibodies were detected with FITC-conjugated anti-rabbit (green) and TRITC-conjugated anti-mouse (red) antisera. Insets show high-magnification images of regions indicated by white boxes; high levels Sox-2, Musashi, GFAP and nestin are expressed by a subset of cells within each sphere. To control for nonspecific staining, some spheres from each experiment were stained with normal rabbit IgG (m)and normal mouse IgG (n) followed by the same secondary antibodies. Red and green pictures were taken at 40× magnification and merged (f,i,l,o)using Openlab software.
Figure 5
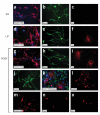
Prominin+lin- cells exhibit multipotency in vitro.(a-k)Generation of neurons, astrocytes and oligodendrocytes. Prominin+lin- cells were cultured at clonal density for 10 d to generate neurospheres. Neurospheres were transferred onto PDL-coated coverslips and cultured in the absence of bFGF and EGF and in the presence of all-trans retinoic acid (RA; a-c), leukemia inhibitory factor (LIF; d-f) or platelet-derived growth factor (PDGF-AA; g-k). After another 7 d, cultures were fixed and stained with antibodies specific for NG2 and S100β (red and blue, respectively; a,d,g), Map-2 (b,e,h), O4 (c,f,i) or GFAP (j). Also shown (k) is a single neurosphere cultured in PDGF-AA and stained with antibodies to O4 (red), Map-2 (green) and S100β (blue). (l-o) Generation of granule and non-granule neurons. Neurospheres differentiated in the presence of PDGF-AA were fixed and stained with antibodies specific for GABA (l), glutamate (m), and Pax-2 (n) and Zic-1 (o) followed by rhodamine-conjugated secondary antibodies. All pictures were taken at 40× magnification and processed using Openlab software.
Figure 6
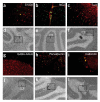
Prominin+lin- cells differentiate into neurons and glia after transplantation into the cerebellum. Freshly isolated prominin+lin- cells were labeled with CM-DiI (red) and injected into the cerebellum of 3-d-old mice (10,000 cells per host). After 2-3 weeks, host cerebella were fixed and stained with antibodies specific for S100β (a), NG2 (b)or TuJ1(c). Primary antibodies were detected with FITC-labeled antisera (green). Double-labeled cells (orange-yellow in a-c) were identified and photographed at 40 magnification; 10× bright-field pictures (d-f) indicate the location of × transplanted cells in the corresponding fluorescent panels. PCL, Purkinje cell layer; IGL, internal granule layer; ML, molecular layer.
Figure 7
Neurospheres derived from a single stem cell can generate neurons and glia in vivo. Prominin+lin- cells from actin-CFP transgenic mice were cultured at clonal density for 10 d to generate neurospheres. Neurospheres were injected into the cerebellum of 3-d-old mice (one neurosphere per host). After 2-3 weeks, host cerebella were fixed and stained with antibodies specific for S100β (a), NG2 (b), TuJ1 (c), GABAA receptor α6(g), parvalbumin (h) or calbindin (i). These antibodies were detected with TRITCconjugated secondary antibodies (red). Chicken antibodies to GFP and FITCconjugated anti-chicken antiserum were used to amplify the CFP signal (green). Double-labeled cells (orange-yellow in a-c and g-i) were identified and photographed at 40× magnification; 10 bright-field pictures (d-f and j-l) indicate the location of transplanted cells×in the corresponding fluorescent panels. WM, white matter; pia, pial membrane surrounding surface of cerebellum; ML, molecular layer; IGL, internal granule layer; PCL, Purkinje cell layer.
Comment in
- Subtracting the Math: prominin-positive cerebellar stem cells in white matter.
Kenney AM, Segal RA. Kenney AM, et al. Nat Neurosci. 2005 Jun;8(6):699-701. doi: 10.1038/nn0605-699. Nat Neurosci. 2005. PMID: 15917830 No abstract available.
Similar articles
- Subtracting the Math: prominin-positive cerebellar stem cells in white matter.
Kenney AM, Segal RA. Kenney AM, et al. Nat Neurosci. 2005 Jun;8(6):699-701. doi: 10.1038/nn0605-699. Nat Neurosci. 2005. PMID: 15917830 No abstract available. - Potential of progenitors from postnatal cerebellar neuroepithelium and white matter: lineage specified vs. multipotent fate.
Milosevic A, Goldman JE. Milosevic A, et al. Mol Cell Neurosci. 2004 Jun;26(2):342-53. doi: 10.1016/j.mcn.2004.02.008. Mol Cell Neurosci. 2004. PMID: 15207858 - Expression of distinct splice variants of the stem cell marker prominin-1 (CD133) in glial cells.
Corbeil D, Joester A, Fargeas CA, Jászai J, Garwood J, Hellwig A, Werner HB, Huttner WB. Corbeil D, et al. Glia. 2009 Jun;57(8):860-74. doi: 10.1002/glia.20812. Glia. 2009. PMID: 19053060 - The heterogeneity of adult neural stem cells and the emerging complexity of their niche.
Alvarez-Buylla A, Kohwi M, Nguyen TM, Merkle FT. Alvarez-Buylla A, et al. Cold Spring Harb Symp Quant Biol. 2008;73:357-65. doi: 10.1101/sqb.2008.73.019. Epub 2008 Nov 6. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19022766 Review. - Origin, lineage and function of cerebellar glia.
Buffo A, Rossi F. Buffo A, et al. Prog Neurobiol. 2013 Oct;109:42-63. doi: 10.1016/j.pneurobio.2013.08.001. Epub 2013 Aug 25. Prog Neurobiol. 2013. PMID: 23981535 Review.
Cited by
- Emerging roles of prominin-1 (CD133) in the dynamics of plasma membrane architecture and cell signaling pathways in health and disease.
Pleskač P, Fargeas CA, Veselska R, Corbeil D, Skoda J. Pleskač P, et al. Cell Mol Biol Lett. 2024 Mar 26;29(1):41. doi: 10.1186/s11658-024-00554-0. Cell Mol Biol Lett. 2024. PMID: 38532366 Free PMC article. Review. - Hindbrain boundaries as niches of neural progenitor and stem cells regulated by the extracellular matrix proteoglycan chondroitin sulphate.
Hutchings C, Nuriel Y, Lazar D, Kohl A, Muir E, Genin O, Cinnamon Y, Benyamini H, Nevo Y, Sela-Donenfeld D. Hutchings C, et al. Development. 2024 Feb 15;151(4):dev201934. doi: 10.1242/dev.201934. Epub 2024 Feb 13. Development. 2024. PMID: 38251863 Free PMC article. - Marinopyrrole derivative MP1 as a novel anti-cancer agent in group 3 MYC-amplified Medulloblastoma.
Coulter DW, Chhonker YS, Kumar D, Kesherwani V, Aldhafiri WN, McIntyre EM, Alexander G, Ray S, Joshi SS, Li R, Murry DJ, Chaturvedi NK. Coulter DW, et al. J Exp Clin Cancer Res. 2024 Jan 11;43(1):18. doi: 10.1186/s13046-024-02944-w. J Exp Clin Cancer Res. 2024. PMID: 38200580 Free PMC article. - Cancer stem cells: a target for overcoming therapeutic resistance and relapse.
Zhang S, Yang R, Ouyang Y, Shen Y, Hu L, Xu C. Zhang S, et al. Cancer Biol Med. 2023 Dec 29;20(12):985-1020. doi: 10.20892/j.issn.2095-3941.2023.0333. Online ahead of print. Cancer Biol Med. 2023. PMID: 38164743 Free PMC article. Review. - β-arrestin1-E2F1-ac axis regulates physiological apoptosis and cell cycle exit in cellular models of early postnatal cerebellum.
Abballe L, Alfano V, Antonacci C, Cefalo MG, Cacchione A, Del Baldo G, Pezzullo M, Po A, Moretti M, Mastronuzzi A, De Smaele E, Ferretti E, Locatelli F, Miele E. Abballe L, et al. Front Cell Dev Biol. 2023 Feb 27;11:990711. doi: 10.3389/fcell.2023.990711. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 36923256 Free PMC article.
References
- Rapoport M, van Reekum R, Mayberg H. The role of the cerebellum in cognition and behavior: a selective review. J. Neuropsychiatry Clin. Neurosci. 2000;12:193–198. - PubMed
- Altman J, Bayer SA. Relation to Its Evolution, Structure and Functions. CRC Press, Boca Raton; Florida, USA: 1997. Development of the Cerebellar System.
- Rosenberg RN, Grossman A. Hereditary ataxia. Neurol. Clin. 1989;7:25–36. - PubMed
- Kern JK. The possible role of the cerebellum in autism/PDD: disruption of a multi-sensory feedback loop. Med. Hypotheses. 2002;59:255–260. - PubMed
- Martin P, Albers M. Cerebellum and schizophrenia: a selective review. Schizophr. Bull. 1995;21:241–250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH067916-01A1/MH/NIMH NIH HHS/United States
- R01 MH067916-05/MH/NIMH NIH HHS/United States
- R01 MH067916/MH/NIMH NIH HHS/United States
- 1R01MH067916-01/MH/NIMH NIH HHS/United States
- R01 MH067916-03/MH/NIMH NIH HHS/United States
- R01 MH067916-04/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials