The ELAV RNA-stability factor HuR binds the 5'-untranslated region of the human IGF-IR transcript and differentially represses cap-dependent and IRES-mediated translation - PubMed (original) (raw)
The ELAV RNA-stability factor HuR binds the 5'-untranslated region of the human IGF-IR transcript and differentially represses cap-dependent and IRES-mediated translation
Zheng Meng et al. Nucleic Acids Res. 2005.
Abstract
The type I insulin-like growth factor receptor (IGF-IR) is an integral component in the control of cell proliferation, differentiation and apoptosis. The IGF-IR mRNA contains an extraordinarily long (1038 nt) 5'-untranslated region (5'-UTR), and we have characterized a diverse series of proteins interacting with this RNA sequence which may provide for intricate regulation of IGF-IR gene expression at the translational level. Here, we report the purification and identification of one of these IGF-IR 5'-UTR-binding proteins as HuR, using a novel RNA crosslinking/RNase elution strategy. Because HuR has been predominantly characterized as a 3'-UTR-binding protein, enhancing mRNA stability and generally increasing gene expression, we sought to determine whether HuR might serve a different function in the context of its binding the IGF-IR 5'-UTR. We found that HuR consistently repressed translation initiation through the IGF-IR 5'-UTR. The inhibition of translation by HuR was concentration dependent, and could be reversed in trans by addition of a fragment of the IGF-IR 5'-UTR containing the HuR binding sites as a specific competitor, or abrogated by deletion of the third RNA recognition motif of HuR. We determined that HuR repressed translation initiation through the IGF-IR 5'-UTR in cells as well, and that siRNA knockdown of HuR markedly increased IGF-IR protein levels. Interestingly, we also found that HuR potently inhibited IGF-IR translation mediated through internal ribosome entry. Kinetic assays were performed to investigate the mechanism of translation repression by HuR and the dynamic interplay between HuR and the translation apparatus. We found that HuR, occupying a cap-distal position, significantly delayed translation initiation mediated by cap-dependent scanning, but was eventually displaced from its binding site, directly or indirectly, as a consequence of ribosomal scanning. However, HuR perpetually blocked the activity of the IGF-IR IRES, apparently arresting the IRES-associated translation pre-initiation complex in an inactive state. This function of HuR as a 5'-UTR-binding protein and dual-purpose translation repressor may be critical for the precise regulation of IGF-IR expression essential to normal cellular homeostasis.
Figures
Figure 1
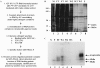
RNA crosslinking/RNase elution strategy for purification and identification of the regulatory proteins binding the IGF-IR 5′-UTR. (A) Diagram of the essential features of the protocol developed and used to enrich and identify RNA-binding proteins potentially regulating IGF-IR expression at the translational level (see Materials and Methods for details). (B) The silver-stained SDS–PAGE gel from a medium-scale preparative trial reveals the high degree of enrichment for the specific RNA-binding proteins relative to total cellular protein attained using this protocol. FT, flowthrough; W1, wash #1 (50 vol binding buffer); W2, wash #2 (containing 2% SDS); W3, wash #3 (containing 1 M KCl); E, elution (RNase A in binding buffer). The positions of the 38 and 42 kDa proteins (in the eluate) which bind specifically to the IGF-IR 5′-UTR [as previously described (41)] are indicated to the right. The positions of the molecular weight markers are indicated to the left. The band seen just below the 38 kDa band was determined to be a contaminant of the RNase (observed with RNase alone). (C) Autoradiograph of an unstained gel (as was used to guide excision of the bands for tandem mass spectrometric analysis) demonstrating the IGF-IR 5′-UTR-binding proteins. N, negative control (no UV crosslinking); P, positive control (input), a small aliquot of the sample, removed before loading on the matrix. The positions of the 38 and 42 kDa proteins are indicated by the arrows on the right. [Note that the HuR–oligoribonucleotide complex (38 kDa) was actually a relatively low-intensity band in the crosslinking/label transfer reaction. Identification of the more predominant 42 kDa crosslinked band will be reported in a separate communication.] The position of the inadvertently released streptavidin tetramer (∼60 kDa, complexed with remnant oligoribonucleotides) is indicated as well.
Figure 2
Confirmation that the 38 kDa IGF-IR 5′-UTR-binding protein is HuR. (A) Specific immunoprecipitation of the 38 kDa UV-crosslinked band by a monoclonal antibody to HuR. Lane 1 is a representative result of the crosslinking/label transfer procedure demonstrating two of the proteins binding specifically to the intact IGF-IR 5′-UTR RNA. In lane 2, the products of the UV crosslinking reaction were subjected to immunoprecipitation using a monoclonal antibody to HuR (3A2) in the presence of Empigen (1%). An isotype-matched irrelevant antibody used as a control yielded a negative result (data not shown). Note that the more predominant 42 kDa band was not co-immunoprecipitated with HuR. (B) Specific binding of recombinant HuR to the IGF-IR 5′-UTR. UV crosslinking was performed following incubation of either purified GST-HuR or GST with the labeled IGF-IR 5′-UTR. GST-HuR (Lane 2), but not GST alone (Lane 1), bound to the IGF-IR 5′-UTR, producing a single ∼62 kDa band. Lane 3 is the result of silver staining of Lane 2, demonstrating the purity and relative amount of the recombinant GST-HuR preparation. (C) Localization of the HuR binding sites within the IGF-IR 5′-UTR. A series of fragments of the IGF-IR 5′-UTR was tested in the UV crosslinking assay to map the binding sites for recombinant GST-HuR. The UV crosslinking data are shown above. The position of three candidate HuR binding sites (symbolized by ellipses), including two short U-rich sequences and one very long polypyrimidine tract within the 890–1038 nt IGF-IR 5′-UTR sequence, are shown below. (D) Demonstration of binding of cellular HuR to the IGF-IR 5′-UTR by altered mobility on immunoblot (western-shift assay). The IGF-IR 5′-UTR was incubated with nuclear extract, and crosslinking followed by RNase T1 digestion performed under highly stringent conditions as described in Materials and Methods. The products of the crosslinking reaction were separated on SDS–PAGE. Following transfer to a 0.2 μm nitrocellulose membrane, the blot was probed with antibody to HuR (3A2). Lane 3: native HuR protein (not crosslinked to RNA). Lanes 4–8: HuR protein UV crosslinked to increasing amounts of IGF-IR 5′-UTR RNA and digested by RNase T1. Lanes 1 and 2: HuR UV crosslinked to IGF-IR 5′-UTR and digested by RNase T1 and A together or RNase A alone. The relative concentration of the RNA is indicated above each lane, and the positions of native HuR as well as HuR shifted by crosslinking to a remnant of the IGF-IR 5′-UTR RNA target sequence are indicated by arrows.
Figure 3
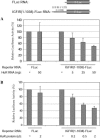
HuR specifically represses translation through the IGF-IR 5′-UTR in a concentration-dependent manner. (A) In vitro co-translation assay. The IGFIR(1–1038)-FLuc RNA (5 ng) was incubated with increasing amounts of in vitro transcribed mRNA encoding HuR (5–50 ng) in an in vitro translation reaction (with RRL). The FLuc RNA (5 ng) was used as a control. The results are expressed relative to the control reactions without HuR. (B) In vitro translation with recombinant HuR protein. HuR protein (no tag) was synthesized in vitro as described in Materials and Methods. Increasing amounts of HuR protein were included in the in vitro translation assay using the IGFIR(1–1038)-FLuc reporter RNA. HuR protein progressively decreased translation of the reporter RNA containing the full-length IGF-IR 5′-UTR but, even at the highest concentration used, had no significant effect on translation of the control luciferase reporter RNA.
Figure 4
Translational repression by HuR is accomplished through direct interaction with its binding sites within the IGF-IR 5′-UTR and does not involve permanent alteration of the RNA. (A) In vitro translation assays were performed using the IGF-IR 5′-UTR reporter and recombinant HuR as described above, except that in these experiments, an isolated 149 nt RNA fragment (nt 890–1038) of the IGF-IR 5′-UTR, which includes all the high-affinity HuR binding sites (refer to Figure 2C), was included as a specific competitor for HuR binding and translational repression. The specific competitor RNA relieved the inhibitory effect of HuR on translation through the IGF-IR 5′-UTR in a concentration-dependent manner. In contrast, another similarly sized fragment of the native IGF-IR 5′-UTR (nt 1–205), which is devoid of HuR binding sites, had no effect on HuR translational repression. The last two bars represent addition of specific competitor RNA in the absence of HuR protein. The results rule out a general stimulatory effect of the specific competitor RNA on translation. Renilla RNA was included in all reactions as an internal control, and all firefly luciferase levels were normalized to Renilla luciferase levels. The results are expressed relative to the control reaction without HuR or competitor RNA. (B) Re-translation assay designed to test the effect of HuR on mRNA stability in vitro. IGFIR(1–1038)-FLuc RNA containing the full length IGF-IR 5′-UTR was incubated with or without HuR in an in vitro translation reaction. Following the first round of translation, RNAs were recovered by phenol/chloroform extraction and ethanol precipitation and were separately used as templates for a second round of in vitro translation in RRL. The results of the re-translation assay demonstrate that the prior incubation of the RNA with HuR has no residual effect on translation efficiency. This experiment was repeated using GST-HuR with essentially identical results.
Figure 5
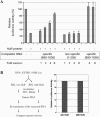
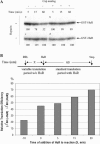
RRM3 is required for HuR translational repression. Full-length HuR and four C-terminal deletion mutants were prepared as described in Materials and Methods. The diagram above indicates the relative positions of the C-termini for each of the HuR truncation mutants. In mutA, RRM3 is selectively deleted. In mutB, the nuclear–cytoplasmic shuttling sequence is also removed. In mutC, RRM2 is bisected, whereas mutD retains only RRM1. (A) The capacity of each of these HuR truncation mutants to function as a translational repressor was measured by the standard in vitro translation assay [using the capped IGF-IR(890–1038)-FLuc reporter RNA] and compared with the full-length HuR generated in the same manner. Renilla luciferase reporter RNA was included in all reactions as an internal control, and the raw data are included (lightly shaded bars on the right). The solid arrow represents the degree of translational repression associated with wild-type HuR, and the dotted arrow represents the minimal residual translational repression observed with each of the C-terminal HuR truncation mutants, apparently attributable to RRM1 alone. (B) Cap analog (3 mM) was included an aliquot of 3 mM cap analog was included in the translation reaction, to inhibit cap-dependent translation initiation of the IGF-IR(890–1038)-FLuc reporter RNA. Thus, this assay specifically measures HuR repression of IRES-mediated translation (discussed in detail later in text). The double-headed arrow represents the degree of repression of IRES-mediated translation initiation observed with wild-type HuR. Note that the ability of HuR to repress IRES-mediated translation initiation is completely abrogated by deletion of RRM3. The experiment was repeated with nearly identical results. (C) Immunoblot analysis of the full-length HuR and C-terminal truncation mutants. Synthesis and accumulation of comparable quantities of the mutC and mutD proteins, which are not detectable with the 3A2 antibody, was confirmed by analysis of 35S-labeled reaction products (data not shown). (D) Standard crosslinking analysis to measure binding of full-length and C-terminal truncation mutants of HuR to the IGF-IR 5′-UTR. Only the wild-type HuR yields a detectable crosslinked band (lane 2); the loss of RNA-binding activity accompanying deletion of RRM3 correlates well with the loss of translational repression (A and B).
Figure 6
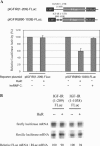
HuR specifically represses translation through the IGF-IR 5′-UTR in cells. (A) The expression plasmid for HuR (200 ng) was co-transfected with pIGFIR(890–1038)-FLuc (containing the HuR binding sites, represented by ellipses in the diagram above) or the control reporter construct [pIGFIR (1–209)-FLuc, no HuR binding sites] (100 ng) into OVCAR3 cells as indicated. Overexpression of HuR was confirmed by western blot analysis (data not shown). Cells were harvested 48 h after transfection and extracts analyzed for firefly luciferase expression. All firefly luciferase activities were normalized for transfection efficiency using a Renilla luciferase control vector. Assays were performed in triplicate and the experiment repeated three times. The results are presented relative to control samples with no ectopically expressed HuR. HuR specifically inhibited translation of the reporter containing its target sequences within the IGF-IR 5′-UTR. The control protein hnRNP C had no such effect. BGHpA: bovine growth hormone polyadenylation site. (B) Confirmation that HuR does not alter the level of reporter mRNA containing the IGF-IR 5′-UTR. Cells were co-transfected with either pIGFIR(1–1038)-FLuc or pIGFIR(1–209)-FLuc (no HuR binding sites), along with the expression plasmid for HuR or pcDNA3.1 (with no insert). The Renilla luciferase control vector was used as an internal control in all samples. Total RNA was harvested 48 h after transfection, and levels of the firefly and Renilla luciferase mRNAs in each cell sample were assessed by RNase protection (as described in Materials and Methods). Firefly luciferase mRNA levels were normalized for Renilla luciferase mRNA levels and quantified relative to that of cells in which HuR was not ectopically expressed.
Figure 7
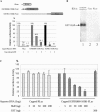
HuR inhibits IRES-mediated translation through the IGF-IR 5′-UTR. A bi-cistronic reporter construct was prepared in which the full-length human IGF-IR 5′-UTR sequence was positioned between the coding sequences for Renilla luciferase (first cistron) and firefly luciferase (second cistron). The bi-cistronic reporter plasmid (100 ng) was co-transfected with different amounts of the HuR expression plasmid (or hnRNP C expression plasmid as control) into OVCAR3 cells as indicated. The total amount of DNA transfected was kept constant by adding pcDNA3 plasmid. At 48 h post-transfection, the firefly and Renilla luciferase activities were measured. The FLuc:RLuc ratio of samples in which HuR or hnRNP C was ectopically expressed is presented relative to control samples with no ectopically expressed HuR. The assays were performed in triplicate and the experiment repeated three times.
Figure 8
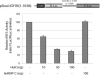
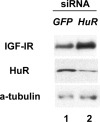
siRNA knockdown of endogenous HuR increases IGF-IR levels in vivo. OVCAR3 cells were transfected with a pool of four siRNA duplexes (final concentration 50 nM) targeted to the human HuR mRNA using Oligofectamine and serum-free medium as described in Materials and Methods. An equivalent concentration of siRNA targeted to EGFP served as a control. Forty-eight hours after transfection, whole-cell lysates were prepared and HuR, IGF-IR and alpha-tubulin levels were assessed by western blot. The experiment was repeated and representative results are shown.
Figure 9
The 890–1038 nt fragment of the IGF-IR 5′-UTR, containing the HuR binding sites and internal ribosome entry window, accurately recapitulates cap-dependent and IRES-mediated translation initiation and HuR repression in vitro. (A) In vitro translation of the control RLuc (Renilla luciferase), full-length IGFIR (1–1038)-FLuc, and IGFIR(890–1038)-FLuc reporter RNA (each capped, 5 ng) was performed in the absence or presence of 1 or 3 mM cap analog. Each of the reporter RNAs is diagrammed schematically at the top. The luciferase activities are expressed as a percentage of the control reactions without cap-analog. (B) Purified recombinant GST-HuR (62 kDa, 10 ng) was incubated with the [32P]UTP-labeled IGFIR(890–1038)-FLuc, FLuc or RLuc reporter RNAs, and UV crosslinking was performed as described in Materials and Methods. Samples were resolved on 10% SDS–PAGE and visualized by autoradiography. The position of the crosslinked GST-HuR band is indicated by an arrow. (C) In vitro translation of the IGFIR(890–1038)-FLuc reporter RNA (5 ng) with the internal control RLuc RNA (1 ng) was performed with increasing concentrations of GST-HuR (or GST as a control). The amount of HuR included in each reaction is indicated. The raw data for both the IGFIR(890–1038)-FLuc and the internal control RLuc RNAs are shown. The luciferase activities are expressed relative to the control reaction without GST-HuR. These experiments were repeated at least three times.
Figure 10
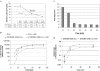
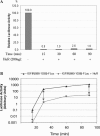
Variability of HuR translational repression over time. (A) Standard in vitro translation assays were performed using the IGFIR(890–1038)-FLuc reporter RNA and variable concentrations of GST-HuR as indicated. The firefly luciferase activities were normalized using the internal control Renilla luciferase RNA. Values obtained using reaction times of 10 or 90 min were compared. The results are expressed as a percentage relative to reactions without HuR, and the numerical data are presented below the graph. (B) In vitro translation assays were performed using the IGFIR(890–1038)-FLuc RNA (5 ng) and the internal control Renilla luciferase RNA (1 ng) with or without GST-HuR (200 ng). The reactions were stopped and luciferase activities measured at 10, 15, 20, 30, 45, 60 and 90 min. The cumulative firefly and Renilla luciferase activities in arbitrary units are plotted on a logarithmic scale. (C) The data from (B) are expressed as fold repression owing to HuR as a function of time. Firefly luciferase readings were normalized to the internal control Renilla luciferase measurements. (D) The data from (B) were re-analyzed to assess the relative rate of protein synthesis (change in luciferase activity per unit of time, translation velocity). Calculations were performed as described in Materials and Methods. Data are plotted on a logarithmic scale (arbitrary units), and the degree of translation repression attributable to HuR is represented by the double-headed arrows. The experiment was repeated with essentially identical results.
Figure 11
HuR potently and persistently represses IGF-IR IRES-mediated translation initiation in vitro. In vitro translation assays were performed in the presence of cap analog (3 mM) using the IGFIR(890–1038)-FLuc reporter RNA (5 ng) with or without GST-HuR (200 ng). Individual translation reactions were stopped at various time points as indicated and the luciferase activities were measured. (A) The luciferase activities of samples with HuR are expressed relative to those of paired control samples without HuR at each corresponding time point. (For clarity, the paired control samples = 100% for each time point are not shown.) (B) Cumulative protein synthesis over time is assessed. Note that a two log decrease in efficiency of IRES-mediated translation in the presence of HuR is maintained throughout the course of the experiment.
Figure 12
HuR is actively displaced from its binding sites on the IGF-IR 5′-UTR by the cap-dependent scanning 43S ribosome complex but not by IRES-mediated translation initiation, and the effectiveness of HuR as a translational repressor is decreased if it does not occupy its binding sites before commencement of active translation initiation. (A) UV crosslinking was utilized to assess HuR binding to the IGF-IR 5′-UTR under conditions conducive to active in vitro translation. Standard in vitro translation assays were performed using the [32P]-UTP labeled IGFIR(890–1038)-FLuc reporter RNA (5 ng), with GST-HuR (200 ng) included in all samples. Cap analog (3 mM) was included in the samples shown in panels b and d. After in vitro translation at 30°C for 5, 15 or 60 min, the samples were immediately transferred to ice. For the samples in panels c and d, heparin (5 mg/ml) was added at this step. Then the samples were exposed to UV irradiation for 30 min on ice, followed by digestion with RNases A and T1. Equal aliquots of the samples were resolved by SDS–PAGE. The results were obtained by autoradiography. The GST-HuR bands are indicated by arrows. Band intensities were quantified using ScionImage (NIH Image) and expressed (as percentages) relative to the 5 min time point. The experiment was repeated three times and a representative result is shown. (B) A two-phase time-course experiment was performed to assess the temporal relationship between HuR RNA-binding and translational repression. The experimental procedure is diagrammed above. An in vitro translation reaction using the IGFIR(890–1038)-FLuc reporter RNA (5 ng) was begun at time 0. Following a variable incubation period (X = 0, 5, 15 or 30 min), GST-HuR (200 ng) was added to the reaction, and the incubation continued for an additional standard 60 min period. The luciferase activities attributable to the standard 60 min translation period following HuR addition (_L_60) were determined by subtracting the luciferase activities attributable to the variable translation period (measured in a series of parallel control samples, LX) from the total cumulative luciferase activities at time X + 60 (LX+60). The relative translation efficiency in the presence of HuR during the standard 60 min period was compared with that of another set of samples to which HuR was never added. The result [_L_60(+HuR)/_L_60(−HuR)] is expressed graphically below. For the sample denoted −10 min, HuR was pre-incubated with the reporter RNA for 10 min prior to addition of RRL.
Figure 13
A two-component model for HuR translation repression through the IGF-IR 5′-UTR. The top section depicts the mechanism through which HuR delays translation initiation by the cap-dependent 43S scanning ribosome complex. In Step 1, HuR (blue oval) binds the IGF-IR 5′-UTR, just upstream of the coding sequence (green rectangle). This does not prevent the recruitment of the 43S translation pre-initiation complex (green ellipse) to the cap structure (red circle) through interaction with the eIF4F complex (orange cylinder). In Step 2, the progress of the scanning 43S complex is impeded as a consequence of HuR binding to the 5′-UTR. In Step 3, after a pause, the scanning 43S complex actively displaces HuR from its binding sites, and proceeds to productive translation initiation. It appears that, once HuR is displaced, active scanning of the 5′-UTR perpetually interferes with re-association of HuR with the IGF-IR RNA. The bottom section illustrates three steps at which HuR could block IRES-mediated translation initiation. In Step 1, HuR may interfere with the initial binding of ITAFs to the 5′-UTR. In Step 2, HuR may permit the association of ITAFs with the 5′-UTR, but prevent the ITAFs from recruiting the 43S ribosome complex. In Step 3, HuR may allow formation of the full IRES-associated pre-initiation complex, but block productive translation initiation. We suspect that either Step 2 or 3 is the critical point at which HuR blocks IRES function, because the arrested partial or complete IRES-associated complex, not HuR alone, apparently then becomes resistant to displacement by the cap-dependent scanning ribosome complex.
Similar articles
- Insulin-like growth factor-I receptor is suppressed through transcriptional repression and mRNA destabilization by a novel energy restriction-mimetic agent.
Chu PC, Kulp SK, Chen CS. Chu PC, et al. Carcinogenesis. 2013 Dec;34(12):2694-705. doi: 10.1093/carcin/bgt251. Epub 2013 Jul 16. Carcinogenesis. 2013. PMID: 23864387 Free PMC article. Retracted. - UNR translation can be driven by an IRES element that is negatively regulated by polypyrimidine tract binding protein.
Cornelis S, Tinton SA, Schepens B, Bruynooghe Y, Beyaert R. Cornelis S, et al. Nucleic Acids Res. 2005 May 31;33(10):3095-108. doi: 10.1093/nar/gki611. Print 2005. Nucleic Acids Res. 2005. PMID: 15928332 Free PMC article. - Properties of the regulatory RNA-binding protein HuR and its role in controlling miRNA repression.
Meisner NC, Filipowicz W. Meisner NC, et al. Adv Exp Med Biol. 2010;700:106-23. Adv Exp Med Biol. 2010. PMID: 21627034 Review. - Protein synthesis in eukaryotes: the growing biological relevance of cap-independent translation initiation.
López-Lastra M, Rivas A, Barría MI. López-Lastra M, et al. Biol Res. 2005;38(2-3):121-46. doi: 10.4067/s0716-97602005000200003. Biol Res. 2005. PMID: 16238092 Review.
Cited by
- Posttranscriptional regulation of cancer traits by HuR.
Abdelmohsen K, Gorospe M. Abdelmohsen K, et al. Wiley Interdiscip Rev RNA. 2010 Sep-Oct;1(2):214-29. doi: 10.1002/wrna.4. Epub 2010 May 6. Wiley Interdiscip Rev RNA. 2010. PMID: 21935886 Free PMC article. Review. - SRI-42127, a novel small molecule inhibitor of the RNA regulator HuR, potently attenuates glial activation in a model of lipopolysaccharide-induced neuroinflammation.
Chellappan R, Guha A, Si Y, Kwan T, Nabors LB, Filippova N, Yang X, Myneni AS, Meesala S, Harms AS, King PH. Chellappan R, et al. Glia. 2022 Jan;70(1):155-172. doi: 10.1002/glia.24094. Epub 2021 Sep 17. Glia. 2022. PMID: 34533864 Free PMC article. - Translational Dysregulation in Cancer: Molecular Insights and Potential Clinical Applications in Biomarker Development.
Vaklavas C, Blume SW, Grizzle WE. Vaklavas C, et al. Front Oncol. 2017 Jul 26;7:158. doi: 10.3389/fonc.2017.00158. eCollection 2017. Front Oncol. 2017. PMID: 28798901 Free PMC article. Review. - Unveil cis-acting combinatorial mRNA motifs by interpreting deep neural network.
Zeng X, Wei Z, Du Q, Li J, Xie Z, Wang X. Zeng X, et al. Bioinformatics. 2024 Jun 28;40(Suppl 1):i381-i389. doi: 10.1093/bioinformatics/btae262. Bioinformatics. 2024. PMID: 38940172 Free PMC article. - Long noncoding RNA ASB16-AS1 inhibits adrenocortical carcinoma cell growth by promoting ubiquitination of RNA-binding protein HuR.
Long B, Yang X, Xu X, Li X, Xu X, Zhang X, Zhang S. Long B, et al. Cell Death Dis. 2020 Nov 20;11(11):995. doi: 10.1038/s41419-020-03205-2. Cell Death Dis. 2020. PMID: 33219221 Free PMC article.
References
- Ullrich A., Gray A., Tam A.W., Yang-Feng T., Tsubokawa M., Collins C., Henzel W., Le Bon T., Kathuria S., Chen E., et al. Insulin-like growth factor I receptor primary structure: comparison with insulin receptor suggests structural determinants that define functional specificity. EMBO J. 1986;5:2503–2512. - PMC - PubMed
- LeRoith D. Insulin-like growth factor I receptor signaling—overlapping or redundant pathways? Endocrinology. 2000;141:1287–1288. - PubMed
- Valentinis B., Romano G., Peruzzi F., Morrione A., Prisco M., Soddu S., Cristofanelli B., Sacchi A., Baserga R. Growth and differentiation signals by the insulin-like growth factor 1 receptor in hemopoietic cells are mediated through different pathways. J. Biol. Chem. 1999;274:12423–12430. - PubMed
- Butler A.A., Yakar S., Gewolb I.H., Karas M., Okubo Y., LeRoith D. Insulin-like growth factor-I receptor signal transduction: at the interface between physiology and cell biology. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998;121:19–26. - PubMed
- Liu J.L., LeRoith D. Insulin-like growth factor I is essential for postnatal growth in response to growth hormone. Endocrinology. 1999;140:5178–5184. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous