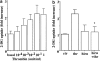Thrombin-induced glucose transport via Src-p38 MAPK pathway in vascular smooth muscle cells - PubMed (original) (raw)
Thrombin-induced glucose transport via Src-p38 MAPK pathway in vascular smooth muscle cells
Yasunari Kanda et al. Br J Pharmacol. 2005 Sep.
Abstract
Thrombin is a mitogen for vascular smooth muscle cells (VSMC) and has been implicated in the development in atherosclerosis. However, little is known about the role of thrombin in glucose transport in VSMC. In this study, we examined the effect of thrombin on glucose uptake in rat A10 VSMC. We found that thrombin induced glucose uptake in a dose-dependent manner while hirudin, a potent thrombin inhibitor, prevented glucose uptake in the cells. PP2, a selective inhibitor of Src, prevented the thrombin-induced glucose uptake, but did not affect insulin-induced uptake. We also examined whether mitogen-activated protein kinase (MAPK) inhibitors influenced thrombin-induced glucose uptake. The p38 MAPK inhibitor (SB203580) inhibited thrombin-induced glucose uptake, but the MEK inhibitor (PD98059) did not. In contrast to thrombin, SB203580 did not affect insulin-induced glucose uptake. Furthermore, thrombin failed to translocate the insulin-sensitive glucose transporter GLUT4. These findings suggest that thrombin stimulates glucose transport via Src and subsequent p38 MAPK activation in VSMC.
Figures
Figure 1
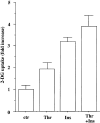
Effect of thrombin on 2-DG uptake in A10 cells. A10 cells in 24-well plates were serum-starved for 24 h. The cells were stimulated for 30 min with various concentrations of thrombin (a) or with 1 U ml−1 of thrombin in the presence or absence of hirudin (b), and were evaluated by assay for 2-DG uptake, as described under Methods. Each value represents the mean±s.d. of three independent experiments in triplicate. *P<0.05 as compared with the respective control.
Figure 2
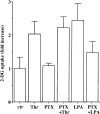
Effect of PTX on thrombin-induced 2-DG uptake in A10 cells. A10 cells were incubated with or without PTX (50 ng ml−1) for 24 h in serum-free DMEM. The cells were stimulated with thrombin (1 U ml−1) or LPA (10 μ
M
) for 30 min and were evaluated by assay for the 2-DG uptake, as described under Methods. Each value represents the mean±s.d. of three independent experiments in triplicate.
Figure 3
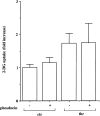
Effects of phosducin on thrombin-induced 2-DG uptake in A10 cells. A10 cells were infected with phosducin and incubated for 24 h. After the cells were serum-starved for further 24 h, the cells were stimulated with thrombin (1 U ml−1) for 30 min and were evaluated by assay for the 2-DG uptake as described under Methods. Each value represents the mean±s.d. of three independent experiments in triplicate.
Figure 4
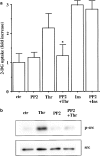
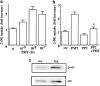
Effects of PP2 on thrombin-induced 2-DG uptake and Src phosphorylation in A10 cells. Serum-starved A10 cells were incubated with or without PP2 (1 μ
M
) for 30 min. (a) The cells were stimulated with thrombin (1 U ml−1) or insulin (1 μ
M
) for 30 min and were evaluated by assay for the 2-DG uptake, as described under Methods. Each value represents the mean±s.d. of three independent experiments in triplicate. *P<0.05 as compared with the respective control. (b) The cells were stimulated with thrombin (1 U ml−1) for 5 min and lysed. Src phosphorylation was analyzed by immunoblotting with anti-phospho-specific Src antibody.
Figure 5
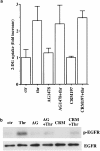
Effects of AG1478 and CRM197 on thrombin-induced 2-DG uptake and EGFR phosphorylation in A10 cells. Serum-starved A10 cells were incubated with or without PP2 (1 μ
M
) for 30 min. (a) The cells were stimulated with thrombin (1 U ml−1) or insulin (1 μ
M
) for 30 min and were evaluated by assay for the 2-DG uptake, as described under Methods. Each value represents the mean±s.d. of three independent experiments in triplicate. (b) The cells were stimulated with thrombin (1 U ml−1) for 5 min and lysed. After immunoprecipitation with anti-EGFR antibody, the samples were analyzed by immunoblotting with anti-phospho-tyrosine antibody or anti-EGFR antibody.
Figure 6
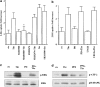
Role of G_α_q in 2-DG uptake in A10 cells. (a) A10 cells were exposed to various concentrations of PMT for 16 h in serum-free DMEM. (b) A10 cells were exposed to PMT for 16 h and then incubated with or without PP2 (1 μ
M
) for 30 min. The cells were evaluated by assay for the 2-DG uptake, as described under Methods. Each value represents the mean±s.d. of three independent experiments in triplicate. *P<0.05 as compared with the respective control. (c) A10 cells were transfected with constitutively active mutant of G_α_q. After 48 h, the cells were lysed and immunoblotted with anti-phospho-specific Src antibody.
Figure 7
Role of p38 MAPK on thrombin-induced 2-DG uptake in A10 cells. Serum-starved A10 cells were stimulated with thrombin (1 U ml−1) or insulin (1 μ
M
) in the presence or absence of (a) SB203580 (10 μ
M
) and SB202474 (10 μ
M
), (b) PD98059 (10 μ
M
) and U0126 (1 μ
M
). The cells were evaluated by assay for the 2-DG uptake, as described under Methods. Each value represents the mean±s.d. of three independent experiments in triplicate. *P<0.05 as compared with the respective control. (c) Serum-starved A10 cells were incubated with or without PD98059 (10 μ
M
) for 30 min and then stimulated with thrombin (1 U ml−1) for 5 min. ERK phosphorylation was analyzed by immunoblotting with anti-phospho-specific ERK antibody. (d) Serum-starved A10 cells were incubated with or without PP2 (1 μ
M
) for 30 min and then stimulated with thrombin (1 U ml−1) for 10 min. p38 MAPK activity was assayed, as described under Methods.
Figure 8
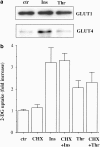
Thrombin did not translocate GLUT4 in A10 cells. (a) Serum-starved A10 cells were stimulated with thrombin (1 U ml−1) or insulin (1 μ
M
) for 20 min. PM fractions were prepared by centrifugation and immunoblotted with anti-GLUT1 or anti-GLUT4 antibody, as described under Methods. (b) Serum-starved A10 cells were incubated with or without cycloheximide (CHX; 10 μ
M
) for 30 min. The cells were stimulated with thrombin (1 U ml−1) or insulin (1 μ
M
) for 30 min and were evaluated by assay for the 2-DG uptake, as described under Methods. Each value represents the mean±s.d. of three independent experiments performed in triplicate.
Figure 9
Stimulation of 2-DG uptake by thrombin and insulin in A10 cells. Serum-starved A10 cells were stimulated with thrombin (1 U ml−1), insulin (1 μ
M
), and the combination of thrombin plus insulin for 30 min and were evaluated by assay for the 2-DG uptake, as described under Methods. Each value represents the mean±s.d. of three independent experiments in triplicate.
Figure 10
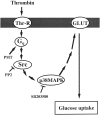
Schematic model summarizing our findings. Src and subsequent p38 MAPK activation plays a role in thrombin-mediated glucose uptake in VSMC.
Similar articles
- Adrenaline increases glucose transport via a Rap1-p38MAPK pathway in rat vascular smooth muscle cells.
Kanda Y, Watanabe Y. Kanda Y, et al. Br J Pharmacol. 2007 Jun;151(4):476-82. doi: 10.1038/sj.bjp.0707247. Epub 2007 Apr 23. Br J Pharmacol. 2007. PMID: 17450172 Free PMC article. - ERK1/2 activation by angiotensin II inhibits insulin-induced glucose uptake in vascular smooth muscle cells.
Izawa Y, Yoshizumi M, Fujita Y, Ali N, Kanematsu Y, Ishizawa K, Tsuchiya K, Obata T, Ebina Y, Tomita S, Tamaki T. Izawa Y, et al. Exp Cell Res. 2005 Aug 15;308(2):291-9. doi: 10.1016/j.yexcr.2005.04.028. Exp Cell Res. 2005. PMID: 15921682 - Role of growth factor receptor transactivation in high glucose-induced increased levels of Gq/11alpha and signaling in vascular smooth muscle cells.
Descorbeth M, Anand-Srivastava MB. Descorbeth M, et al. J Mol Cell Cardiol. 2010 Aug;49(2):221-33. doi: 10.1016/j.yjmcc.2009.12.010. Epub 2009 Dec 28. J Mol Cell Cardiol. 2010. PMID: 20036247 - Urokinase-induced smooth muscle cell responses require distinct signaling pathways: a role for the epidermal growth factor receptor.
Nicholl SM, Roztocil E, Davies MG. Nicholl SM, et al. J Vasc Surg. 2005 Apr;41(4):672-81. doi: 10.1016/j.jvs.2005.01.007. J Vasc Surg. 2005. PMID: 15874933
Cited by
- Characterization of Circular RNAs in Vascular Smooth Muscle Cells with Vascular Calcification.
Ryu J, Kwon DH, Choe N, Shin S, Jeong G, Lim YH, Kim J, Park WJ, Kook H, Kim YK. Ryu J, et al. Mol Ther Nucleic Acids. 2020 Mar 6;19:31-41. doi: 10.1016/j.omtn.2019.11.001. Epub 2019 Nov 14. Mol Ther Nucleic Acids. 2020. PMID: 31790973 Free PMC article. - Thrombin Induces COX-2 and PGE2 Expression via PAR1/PKCalpha/MAPK-Dependent NF-kappaB Activation in Human Tracheal Smooth Muscle Cells.
Yang CC, Hsiao LD, Shih YF, Hsu CK, Hu CY, Yang CM. Yang CC, et al. Mediators Inflamm. 2022 Apr 19;2022:4600029. doi: 10.1155/2022/4600029. eCollection 2022. Mediators Inflamm. 2022. PMID: 35497094 Free PMC article. - Targeting tyrosine kinases and autophagy in prostate cancer.
Kung HJ. Kung HJ. Horm Cancer. 2011 Feb;2(1):38-46. doi: 10.1007/s12672-010-0053-3. Epub 2010 Dec 2. Horm Cancer. 2011. PMID: 21350583 Free PMC article. Review. - Adrenaline increases glucose transport via a Rap1-p38MAPK pathway in rat vascular smooth muscle cells.
Kanda Y, Watanabe Y. Kanda Y, et al. Br J Pharmacol. 2007 Jun;151(4):476-82. doi: 10.1038/sj.bjp.0707247. Epub 2007 Apr 23. Br J Pharmacol. 2007. PMID: 17450172 Free PMC article. - Activation of the non-receptor tyrosine kinase cSrc in macrophage-rich atherosclerotic plaques of human carotid arteries.
Toi S, Shibata N, Sawada T, Kobayashi M, Uchiyama S. Toi S, et al. Acta Histochem Cytochem. 2007 Dec 21;40(6):153-61. doi: 10.1267/ahc.07026. Acta Histochem Cytochem. 2007. PMID: 18224247 Free PMC article.
References
- BROWN M.T., COOPER J.A. Regulation, substrates and functions of src. Biochim. Biophys. Acta. 1996;1287:121–149. - PubMed
- CZECH M.P., CORVERA S. Signaling mechanisms that regulate glucose transport. J. Biol. Chem. 1999;274:1865–1868. - PubMed
- DELLA ROCCA G.J., MAUDSLEY S., DAAKA Y., LEFKOWITZ R.J., LUTTRELL L.M. Pleiotropic coupling of G protein-coupled receptors to the mitogen-activated protein kinase cascade. Role of focal adhesions and receptor tyrosine kinases. J. Biol. Chem. 1999;274:13978–13984. - PubMed
- EGUCHI S., NUMAGUCHI K., IWASAKI H., MATSUMOTO T., YAMAKAWA T., UTSUNOMIYA H., MOTLEY E.D., KAWAKATSU H., OWADA K.M., HIRATA Y., MARUMO F., INAGAMI T. Calcium-dependent epidermal growth factor receptor transactivation mediates the angiotensin II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. J. Biol. Chem. 1998;273:8890–8896. - PubMed
- ESSLER M., HERMANN K., AMANO M., KAIBUCHI K., HEESEMANN J., WEBER P.C., AEPFELBACHER M. Pasteurella multocida toxin increases endothelial permeability via Rho kinase and myosin light chain phosphatase. J. Immunol. 1998;161:5640–5646. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous