Mutations in proline 82 of p53 impair its activation by Pin1 and Chk2 in response to DNA damage - PubMed (original) (raw)
Mutations in proline 82 of p53 impair its activation by Pin1 and Chk2 in response to DNA damage
Michael Berger et al. Mol Cell Biol. 2005 Jul.
Abstract
Tumor suppression by the p53 protein largely depends on the elimination of damaged cells by apoptosis. Mutations in the polyproline region (PPR) of p53 impair its apoptotic function. Deletion of the PPR renders p53 more sensitive to inhibition by Mdm2 via an unknown mechanism. We have explored the mechanism by which the PPR modulates the p53/Mdm2 loop. Proline 82 of p53 was identified to be essential for its interaction with the checkpoint kinase 2 (Chk2) and consequent phosphorylation of p53 on serine 20, following DNA damage. These physical and functional interactions are regulated by Pin1 through cis-trans isomerization of proline 82. Our study unravels the pathway by which Pin1 activates p53 in response to DNA damage and explains how Pin1 protects p53 from Mdm2. Further, we propose a role for Pin1-dependent induction of p53 conformational change as a mechanism responsible for the enhanced interaction between p53 and Chk2 following DNA damage. Importantly, our findings elucidate the selection for mutations in the Pin1 target Thr81/Pro82 motif within the PPR of p53 in human cancer.
Figures
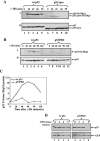
FIG. 1.
Pro82 is important for the efficient and prolonged Ser20 phosphorylation of p53 in response to DNA damage. Saos-2 cells (A) or H1299 cells (B) were transiently transfected with expression plasmids for either wt p53 or p53ΔproAE (A) or for p53P82I (B) (1 μg each). Forty hours posttransfection, cells were exposed to γ-IR (10 Gy) before being harvested at the indicated times. By use of Western blot analysis, phosphorylation (p) of p53 Ser20 was determined with anti-p53-phospho-Ser20 antibodies (I), and the level of p53 expression was determined with a mixture of anti-p53 antibodies (PAb1801 and DO1) (II). (C) The extent of Ser20 phosphorylation (B, panel I**)** was calculated relative to the level of p53 expression (B, panel II**)** using densitometry. The ratios of wt p53 to the p53P82I mutant versus time following IR treatment were plotted on a graph. (D) H1299 cells were transfected and treated as described above, with the exception that cycloheximide (CHX) (10 μg/ml) was added 10 min before IR treatment. Cells were harvested at the indicated times, and the levels of p53 were monitored as described above. The transfection efficiencies were determined by introducing a GFP expression plasmid into each sample and monitoring the expression with anti-GFP antibody.
FIG. 2.
Pro82 facilitates DNA damage-induced p53/Chk2 interaction in vivo but not in vitro. (A) H1299 cells were transfected with expression plasmids for wt p53 or the p53P82I mutant (1 μg each). Forty hours posttransfection, cells were treated with a 300 nM concentration of the phosphatase inhibitor okadaic acid (O.A.) for 2 h (+) or left untreated (−) before being harvested. Serine 20 phosphorylation (p) (I) and p53 expression levels (II) were monitored as described in the legend to Fig. 1. (B) Equal amounts of in vitro-translated proteins of p53 (wt p53, p53P82I, and p53ΔPro) were incubated in vitro with bacterially expressed GST-Chk2 or GST alone followed by a pulldown assay. The amounts of bound p53 proteins were monitored by Western blot analysis using a mixture of anti-p53 antibodies (PAb1801 and DO1) (I). The amounts of input p53 forms and GST-Chk2 were determined by removing aliquots of each sample prior to the pulldown assay and subjecting these to Western blot analysis using anti-p53 antibodies (DO1 and PAb1801) (II) or anti-Chk2 antibody (DCS-273) (III). The binding results between wt p53 and GST and GST-Chk2 without p53 were used as the controls (lanes 4 and 5). (C) 293 cells were transfected with expression plasmids for Chk2 (4 μg) together with wt p53 or p53P82I (2 μg each). Forty hours posttransfection, cells were exposed to IR (10 Gy), and at the times indicated, cell extracts were subjected to an immunoprecipitation (IP) assay using anti-p53 antibody (PAb421), followed by immunoblotting (IB) with anti-Chk2 antibody (DCS-273) (I). The levels of p53 (II) and Chk2 (III) expression in each sample were monitored by removing aliquots of cell extracts prior to immunoprecipitation and subjecting these to Western blot analysis as described above. (D) The intensities of the bound Chk2 and the amounts of p53 shown in panel C were determined by densitometry, and the ratios of bound Chk2 to p53 expression were plotted. The ratio at time zero was taken as 1.
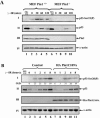
FIG. 3.
Pin1 is essential for Ser20 phosphorylation of p53 in response to DNA damage. (A) MEFs from wt or _Pin1_-null mice were exposed to IR (10 Gy), treated with MG132 (20 μM) for 4 h, or left untreated. At the indicated times, cells were harvested and the levels of Ser23 phosphorylation (P) and p53 expression were determined as described in the legend to Fig. 1. The expression levels of Pin1 were determined with anti-Pin1 polyclonal antibody, and the amount of extract loaded was determined by use of antiactin. (B) A pool of mock-transfected U2OS cells and a pool of stably transfected Ha-Pin1C109A mutants were either left untreated or exposed to IR (20 Gy). At the indicated times, the levels of Ser20 phosphorylation (I) and p53 expression (II) were monitored as described for panel A, while Ha-Pin1 was monitored by anti-Ha antibody (III). As a control, U2OS cells were exposed to the proteasome inhibitor MG132 (20 μM) for 4 h. The amount of extract loaded was determined by reprobing the same membrane with anti-α-actin (IV). O.D., optical density.
FIG. 4.
Pin1 enhances p53/Chk2 interaction in response to DNA damage. (A) H1299 cells were transfected with expression plasmids for p53 (3 μg) and Ha-Pin1 (5 μg). Twenty-four hours posttransfection, cells were exposed to IR (10 Gy) (+) or left untreated (−). Ninety minutes after IR, cell extracts were subjected to a pulldown assay using bacterially expressed GST-Chk2 or GST beads alone. The amounts of bound p53 and input p53 were monitored by immunoblotting (IB) as described in the legend to Fig. 1. (B) H1299 cells were transfected with expression plasmids for p53 alone (3 μg) (−) or together with Ha-Pin1 (5 μg) (+). Twenty-four hours posttransfection, cells were either left untreated or irradiated (10 Gy). At the indicated times, cell extracts were subjected to a pulldown assay using bacterially expressed GST-Chk2 beads. The amount of bound p53 was determined by Western blotting using anti-p53 antibodies (PAb1801 and DO1) (I). The amounts of input p53 (II) and Ha-Pin1 (III) were monitored with anti-p53 and anti-Ha antibodies, respectively. (C) The intensities of the bound p53 and amounts of p53 shown in panel B were determined by densitometry, and the ratios of bound p53 to p53 expression were plotted. The ratio at time zero was taken as 1. (D) H1299 cells were transfected with the indicated expression plasmids. Twenty-four hours posttransfection, cells were either left untreated or irradiated (10 Gy). Ninety minutes after irradiation, cells were subjected to a GST-Chk2 pulldown assay as described for panel A. The levels of input p53 (II) and Ha-Pin1 (III) are shown.
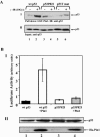
FIG. 5.
Pro82 is essential for binding and activation of p53 by Pin1. (A) H1299 cells were transfected with expression plasmids for either wt p53, the p53P82I mutant, or the p53S33A,S315A,T81A triple mutant (p53T mut). Twenty-four hours posttransfection, cells were either left untreated (−) or irradiated (10 Gy) (+), and 45 min later, cell extracts were subjected to a pulldown assay using GST-Pin1 beads. The amounts of bound p53 (I) and input p53 (II) were determined by Western blot analysis using anti-p53 antibodies (DO1 and PAb1801). (B) U2OS cells were transfected with the indicated expression plasmids (0.1 μg for p53 plasmids and 1 μg for Ha-Pin1 plasmids) together with a luciferase reporter plasmid (0.3 μg) driven by the p21 promoter. Twenty-four hours after transfection, the luciferase activity was determined for each sample (I). The luciferase activity is expressed in arbitrary units, and the averages and standard deviations for three independent experiments are shown. The amounts of p53 and Ha-Pin1 were monitored by Western blot analysis (II) using anti-p53 and anti-Ha antibodies, respectively.
Similar articles
- The prolyl isomerase Pin1 is a regulator of p53 in genotoxic response.
Zheng H, You H, Zhou XZ, Murray SA, Uchida T, Wulf G, Gu L, Tang X, Lu KP, Xiao ZX. Zheng H, et al. Nature. 2002 Oct 24;419(6909):849-53. doi: 10.1038/nature01116. Epub 2002 Oct 2. Nature. 2002. PMID: 12397361 - The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults.
Zacchi P, Gostissa M, Uchida T, Salvagno C, Avolio F, Volinia S, Ronai Z, Blandino G, Schneider C, Del Sal G. Zacchi P, et al. Nature. 2002 Oct 24;419(6909):853-7. doi: 10.1038/nature01120. Epub 2002 Oct 2. Nature. 2002. PMID: 12397362 - Role of Pin1 in the regulation of p53 stability and p21 transactivation, and cell cycle checkpoints in response to DNA damage.
Wulf GM, Liou YC, Ryo A, Lee SW, Lu KP. Wulf GM, et al. J Biol Chem. 2002 Dec 13;277(50):47976-9. doi: 10.1074/jbc.C200538200. Epub 2002 Oct 17. J Biol Chem. 2002. PMID: 12388558 - [Recent advances in the study of pin1 and its inhibitors].
Zhang CJ, Zhang ZH, Xu BL, Wang YL. Zhang CJ, et al. Yao Xue Xue Bao. 2008 Jan;43(1):9-17. Yao Xue Xue Bao. 2008. PMID: 18357725 Review. Chinese. - KeePin' the p53 family in good shape.
Mantovani F, Gostissa M, Collavin L, Del Sal G. Mantovani F, et al. Cell Cycle. 2004 Jul;3(7):905-11. Epub 2004 Jul 2. Cell Cycle. 2004. PMID: 15254434 Review.
Cited by
- Misfolding, Aggregation, and Disordered Segments in c-Abl and p53 in Human Cancer.
de Oliveira GA, Rangel LP, Costa DC, Silva JL. de Oliveira GA, et al. Front Oncol. 2015 Apr 29;5:97. doi: 10.3389/fonc.2015.00097. eCollection 2015. Front Oncol. 2015. PMID: 25973395 Free PMC article. Review. - The prolyl-isomerase Pin1 activates the mitochondrial death program of p53.
Sorrentino G, Mioni M, Giorgi C, Ruggeri N, Pinton P, Moll U, Mantovani F, Del Sal G. Sorrentino G, et al. Cell Death Differ. 2013 Feb;20(2):198-208. doi: 10.1038/cdd.2012.112. Epub 2012 Aug 31. Cell Death Differ. 2013. PMID: 22935610 Free PMC article. - Ser46 phosphorylation of p53 is an essential event in prolyl-isomerase Pin1-mediated p53-independent apoptosis in response to heat stress.
Li L, Su Z, Zou Z, Tan H, Cai D, Su L, Gu Z. Li L, et al. Cell Death Dis. 2019 Feb 4;10(2):96. doi: 10.1038/s41419-019-1316-8. Cell Death Dis. 2019. PMID: 30718466 Free PMC article. - Autoregulatory control of the p53 response by caspase-mediated processing of HIPK2.
Gresko E, Roscic A, Ritterhoff S, Vichalkovski A, del Sal G, Schmitz ML. Gresko E, et al. EMBO J. 2006 May 3;25(9):1883-94. doi: 10.1038/sj.emboj.7601077. Epub 2006 Apr 6. EMBO J. 2006. PMID: 16601678 Free PMC article. - Mutant TP53 posttranslational modifications: challenges and opportunities.
Nguyen TA, Menendez D, Resnick MA, Anderson CW. Nguyen TA, et al. Hum Mutat. 2014 Jun;35(6):738-55. doi: 10.1002/humu.22506. Epub 2014 Feb 11. Hum Mutat. 2014. PMID: 24395704 Free PMC article. Review.
References
- Baptiste, N., P. Friedlander, X. Chen, and C. Prives. 2002. The proline-rich domain of p53 is required for cooperation with anti-neoplastic agents to promote apoptosis of tumor cells. Oncogene 21:9-21. - PubMed
- Bartek, J., and J. Lukas. 2003. Chk1 and Chk2 kinases in checkpoint control and cancer. Cancer Cell 3:421-429. - PubMed
- Berger, M., R. Vogt Sionov, A. J. Levine, and Y. Haupt. 2001. A role for the polyproline domain of p53 in its regulation by Mdm2. J. Biol. Chem. 276:3785-3790. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous