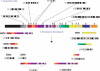Punctuated duplication seeding events during the evolution of human chromosome 2p11 - PubMed (original) (raw)
Comparative Study
Punctuated duplication seeding events during the evolution of human chromosome 2p11
Julie E Horvath et al. Genome Res. 2005 Jul.
Abstract
Primate genomic sequence comparisons are becoming increasingly useful for elucidating the evolutionary history and organization of our own genome. Such studies are particularly informative within human pericentromeric regions--areas of particularly rapid change in genomic structure. Here, we present a systematic analysis of the evolutionary history of one approximately 700-kb region of 2p11, including the first autosomal transition from pericentromeric sequence to higher-order alpha-satellite DNA. We show that this region is composed of segmental duplications corresponding to 14 ancestral segments ranging in size from 4 kb to approximately 115 kb. These duplicons show 94%-98.5% sequence identity to their ancestral loci. Comparative FISH and phylogenetic analysis indicate that these duplicons are differentially distributed in human, chimpanzee, and gorilla genomes, whereas baboon has a single putative ancestral locus for all but one of the duplications. Our analysis supports a model where duplicative transposition events occurred during a narrow window of evolution after the separation of the human/ape lineage from the Old World monkeys (10-20 million years ago). Although dramatic secondary dispersal events occurred during the radiation of the human, chimpanzee, and gorilla lineages, duplicative transposition seeding events of new material to this particular pericentromeric region abruptly ceased after this time period. The multiplicity of initial duplicative transpositions prior to the separation of humans and great-apes suggests a punctuated model for the formation of highly duplicated pericentromeric regions within the human genome. The data further indicate that factors other than sequence are important determinants for such bursts of duplicative transposition from the euchromatin to pericentromeric regions.
Figures
Figure 1.
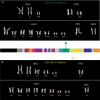
2p11 Duplicon architecture. (A) A schematic representation of the duplicon architecture (colored bars) is shown in reference to an ideogram of chromosome 2 and ∼700-kb BAC minimal tiling path. The black bar represents α-satellite sequence (∼175 kb), while light gray bars denote various pericentromeric-specific interspersed repeats (PIRs). Other enriched pericentromeric repeat sequences are indicated: C=CAAAAAG repeat, G=CAGGG, R=REP522, and T=TAR1 repeats (Smit 1996). Below the BAC tiling path are results of database searches using this entire sequence (represented by NT_034508) against the human genome (build34, July 2003). All pairwise alignments (>5 kb and >90%) to this segment are shown to other regions of the genome as indicated by the chromosome number and approximate position in megabases (ancestral loci are denoted by cytogenetic band position). A color scheme encodes the average percentage sequence identity for each alignment block (red, 99%; orange, 98–99%; yellow, 97–98%; green, 96–97%; blue, 95–96%; indigo, 94–95%; and violet, 90–94%). (B) Sequence overlaps were confirmed by Southern analysis between BAC clone and genomic DNA. An example of validation is shown for overlap D (between AC127391 [R11–389I13] and AC027612 [R11–165D20]). A PCR-generated probe (165D20–6n7) (Supplemental Table 2) was hybridized. The expected 2.2-kb band is observed in multiple overlapping BACs (389I13, 165D20, 34O12, and 1430E12) in addition to the chromosome 2 hybrid and genomic DNA samples. Note: An additional lower band is observed in the genomic DNA samples compared with the monochromosomal hybrid DNA samples, indicating that at least one additional copy of the GGT1 duplicon exists within the human genome. (C) Extended fiber FISH validating overlap (in yellow) of the three most proximal BACs in a chromosome 2 hybrid cell line (GM11712). Results in a second chromosome 2 hybrid line (GM11686) and total human cell lines showed similar results (data not shown).
Figure 1.
2p11 Duplicon architecture. (A) A schematic representation of the duplicon architecture (colored bars) is shown in reference to an ideogram of chromosome 2 and ∼700-kb BAC minimal tiling path. The black bar represents α-satellite sequence (∼175 kb), while light gray bars denote various pericentromeric-specific interspersed repeats (PIRs). Other enriched pericentromeric repeat sequences are indicated: C=CAAAAAG repeat, G=CAGGG, R=REP522, and T=TAR1 repeats (Smit 1996). Below the BAC tiling path are results of database searches using this entire sequence (represented by NT_034508) against the human genome (build34, July 2003). All pairwise alignments (>5 kb and >90%) to this segment are shown to other regions of the genome as indicated by the chromosome number and approximate position in megabases (ancestral loci are denoted by cytogenetic band position). A color scheme encodes the average percentage sequence identity for each alignment block (red, 99%; orange, 98–99%; yellow, 97–98%; green, 96–97%; blue, 95–96%; indigo, 94–95%; and violet, 90–94%). (B) Sequence overlaps were confirmed by Southern analysis between BAC clone and genomic DNA. An example of validation is shown for overlap D (between AC127391 [R11–389I13] and AC027612 [R11–165D20]). A PCR-generated probe (165D20–6n7) (Supplemental Table 2) was hybridized. The expected 2.2-kb band is observed in multiple overlapping BACs (389I13, 165D20, 34O12, and 1430E12) in addition to the chromosome 2 hybrid and genomic DNA samples. Note: An additional lower band is observed in the genomic DNA samples compared with the monochromosomal hybrid DNA samples, indicating that at least one additional copy of the GGT1 duplicon exists within the human genome. (C) Extended fiber FISH validating overlap (in yellow) of the three most proximal BACs in a chromosome 2 hybrid cell line (GM11712). Results in a second chromosome 2 hybrid line (GM11686) and total human cell lines showed similar results (data not shown).
Figure 2.
Comparative primate FISH of individual duplicons. Two examples of comparative metaphase FISH experiments for the (A) IGSF3 (dark green) duplicon from 1p13 and the (B) MLL3 duplicon (in yellow) from 7q36 are shown. Extracted metaphases for five primates are shown after hybridization with probes corresponding to the two duplicons: HSA indicates H. sapiens; PTR, P. troglodytes; GGO, G. gorilla; PPY, P. pygmaeus; and MFA, M. fascicularis. Both sets of experiments show multiple signals among humans and the great-apes with a single signal in the Old World monkey macaque. These results are consistent with the phylogenetic and comparative genomic hybridization experiments that suggest a duplication of the ancestral locus <23 Mya. All chromosomal designations are with respect to the human phylogenetic group (McConkey 2004).
Figure 3.
Phylogenetic trees for 2p11 duplicons. A neighbor-joining tree was constructed for each individual duplicon as shown above and below the gray schematic of the 2p11 duplicons (A_–_K). See Figure 1 for corresponding colored boxes. Gray boxes outline ancestral human, orangutan (Orang), and baboon (Bab) sequence taxa within the phylogenetic trees. Ancestral human sequences are also marked with an arrow. Branch lengths are proportional to the number of nucleotide changes between taxa and are indicated below each respective branch. An asterisk next to or below a branch length indicates a branch length of 0.001. Bootstrap values >90 from 1000 replicates are indicated above each corresponding branch. Sequence data from baboon and orangutan outgroups were obtained from large-insert BAC clones (CHORI-253 and RPCI-41) or total genomic DNA.
Figure 3.
Phylogenetic trees for 2p11 duplicons. A neighbor-joining tree was constructed for each individual duplicon as shown above and below the gray schematic of the 2p11 duplicons (A_–_K). See Figure 1 for corresponding colored boxes. Gray boxes outline ancestral human, orangutan (Orang), and baboon (Bab) sequence taxa within the phylogenetic trees. Ancestral human sequences are also marked with an arrow. Branch lengths are proportional to the number of nucleotide changes between taxa and are indicated below each respective branch. An asterisk next to or below a branch length indicates a branch length of 0.001. Bootstrap values >90 from 1000 replicates are indicated above each corresponding branch. Sequence data from baboon and orangutan outgroups were obtained from large-insert BAC clones (CHORI-253 and RPCI-41) or total genomic DNA.
Figure 4.
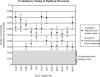
Sequence divergence of 2p11 duplicons. The graph compares the average divergence (substitutions per site, Kimura two-parameter model with standard error measurements) for baboon and all human duplicate copies (circles) to the average divergence for the human ancestral locus to all human pericentromeric copies (triangles). The former provides a locus-specific estimate of the effective number of substitutions since the divergence of Old World monkeys and human lineages (∼23 Mya), while the latter provides an estimate of the timing of the initial duplication event. With the exception of LSP1, the baboon copy corresponds to a single (nonduplicated) locus. The data are consistent with an initial duplicative transposition of the ancestral locus for all loci after separation of the Old World and human lineages. No duplications from an ancestral locus are observed within this 700-kb region which show <0.03 substitutions/per site. This suggests a cessation of euchromatic colonization of this region ∼10 Mya.
Figure 5.
A model for the acquisition and dispersal of 2p11 duplicons. An expanded two-step model is shown to explain the current organization of 2p11. First, a burst of DNA duplicative transposition events occurs in the common ancestor of humans and apes (10–20 Myr), creating a large mosaic region consisting of at least 14 duplicons. During the radiation of humans and African great-apes (4–8 Mya), a series of secondary duplications disperse larger cassettes to other pericentromeric regions, leading to quantitative and qualitative differences of each larger block within different lineages. More recent transposition events suddenly cease or are no longer fixed during this second phase.
Similar articles
- Evolutionary dynamics of segmental duplications from human Y-chromosomal euchromatin/heterochromatin transition regions.
Kirsch S, Münch C, Jiang Z, Cheng Z, Chen L, Batz C, Eichler EE, Schempp W. Kirsch S, et al. Genome Res. 2008 Jul;18(7):1030-42. doi: 10.1101/gr.076711.108. Epub 2008 Apr 29. Genome Res. 2008. PMID: 18445620 Free PMC article. - Using a pericentromeric interspersed repeat to recapitulate the phylogeny and expansion of human centromeric segmental duplications.
Horvath JE, Gulden CL, Bailey JA, Yohn C, McPherson JD, Prescott A, Roe BA, de Jong PJ, Ventura M, Misceo D, Archidiacono N, Zhao S, Schwartz S, Rocchi M, Eichler EE. Horvath JE, et al. Mol Biol Evol. 2003 Sep;20(9):1463-79. doi: 10.1093/molbev/msg158. Epub 2003 May 30. Mol Biol Evol. 2003. PMID: 12777517 - Evolutionary analysis of the highly dynamic CHEK2 duplicon in anthropoids.
Münch C, Kirsch S, Fernandes AM, Schempp W. Münch C, et al. BMC Evol Biol. 2008 Oct 2;8:269. doi: 10.1186/1471-2148-8-269. BMC Evol Biol. 2008. PMID: 18831734 Free PMC article. - Lessons from the human genome: transitions between euchromatin and heterochromatin.
Horvath JE, Bailey JA, Locke DP, Eichler EE. Horvath JE, et al. Hum Mol Genet. 2001 Oct 1;10(20):2215-23. doi: 10.1093/hmg/10.20.2215. Hum Mol Genet. 2001. PMID: 11673404 Review. - Recent duplication, domain accretion and the dynamic mutation of the human genome.
Eichler EE. Eichler EE. Trends Genet. 2001 Nov;17(11):661-9. doi: 10.1016/s0168-9525(01)02492-1. Trends Genet. 2001. PMID: 11672867 Review.
Cited by
- The variant inv(2)(p11.2q13) is a genuinely recurrent rearrangement but displays some breakpoint heterogeneity.
Fickelscher I, Liehr T, Watts K, Bryant V, Barber JC, Heidemann S, Siebert R, Hertz JM, Tumer Z, Simon Thomas N. Fickelscher I, et al. Am J Hum Genet. 2007 Oct;81(4):847-56. doi: 10.1086/521226. Epub 2007 Aug 28. Am J Hum Genet. 2007. PMID: 17847011 Free PMC article. - The Enhancer of split complex arose prior to the diversification of schizophoran flies and is strongly conserved between Drosophila and stalk-eyed flies (Diopsidae).
Baker RH, Kuehl JV, Wilkinson GS. Baker RH, et al. BMC Evol Biol. 2011 Dec 8;11:354. doi: 10.1186/1471-2148-11-354. BMC Evol Biol. 2011. PMID: 22151427 Free PMC article. - Human subtelomeric duplicon structure and organization.
Ambrosini A, Paul S, Hu S, Riethman H. Ambrosini A, et al. Genome Biol. 2007;8(7):R151. doi: 10.1186/gb-2007-8-7-r151. Genome Biol. 2007. PMID: 17663781 Free PMC article. - SNP discovery and haplotype analysis in the segmentally duplicated DRD5 coding region.
Housley DJ, Nikolas M, Venta PJ, Jernigan KA, Waldman ID, Nigg JT, Friderici KH. Housley DJ, et al. Ann Hum Genet. 2009 May;73(Pt 3):274-82. doi: 10.1111/j.1469-1809.2009.00513.x. Epub 2009 Mar 30. Ann Hum Genet. 2009. PMID: 19397556 Free PMC article. - Holocentromeres in Rhynchospora are associated with genome-wide centromere-specific repeat arrays interspersed among euchromatin.
Marques A, Ribeiro T, Neumann P, Macas J, Novák P, Schubert V, Pellino M, Fuchs J, Ma W, Kuhlmann M, Brandt R, Vanzela AL, Beseda T, Šimková H, Pedrosa-Harand A, Houben A. Marques A, et al. Proc Natl Acad Sci U S A. 2015 Nov 3;112(44):13633-8. doi: 10.1073/pnas.1512255112. Epub 2015 Oct 21. Proc Natl Acad Sci U S A. 2015. PMID: 26489653 Free PMC article.
References
- Crosier, M., Viggiano, L., Guy, J., Misceo, D., Stones, R., Wei, W., Hearn, T., Ventura, M., Archidiacono, N., Rocchi, M., et al. 2002. Human paralogs of KIAA0187 were created through independent pericentromeric-directed and chromosome-specific duplication mechanisms. Genome Res. 12: 67-80. - PMC - PubMed
Web site references
- http://humanparalogy.gs.washington.edu/parasight; PARASIGHT.
- http://ftp.genome.washington.edu/RM/RepeatMasker.html; RepeatMasker.
- http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=unigene; UniGene clusters.
- http://www.megasoftware.net/; MEGA.
- http://genome.wustl.edu; PHRED/PHRAP/CONSED software.
Publication types
MeSH terms
Grants and funding
- R01 GM058815/GM/NIGMS NIH HHS/United States
- T32 GM008613/GM/NIGMS NIH HHS/United States
- GM58815/GM/NIGMS NIH HHS/United States
- GM08613/GM/NIGMS NIH HHS/United States
- R01 HG002385/HG/NHGRI NIH HHS/United States
- GTF04001/TI_/Telethon/Italy
- HG002385/HG/NHGRI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous