Therapeutic expression of the platelet-specific integrin, alphaIIbbeta3, in a murine model for Glanzmann thrombasthenia - PubMed (original) (raw)
Therapeutic expression of the platelet-specific integrin, alphaIIbbeta3, in a murine model for Glanzmann thrombasthenia
Juan Fang et al. Blood. 2005.
Abstract
Integrins mediate the adhesion of cells to each other and to the extracellular matrix during development, immunity, metastasis, thrombosis, and wound healing. Molecular defects in either the alpha- or beta-subunit can disrupt integrin synthesis, assembly, and/or binding to adhesive ligands. This is exemplified by the bleeding disorder, Glanzmann thrombasthenia (GT), where abnormalities of the platelet-specific integrin, alphaIIbbeta3, prevent platelet aggregation following vascular injury. We previously used a retrovirus vector containing a cDNA cassette encoding human integrin beta3 to restore integrin alphaIIbbeta3 on the surface of megakaryocytes derived from peripheral blood stem cells of GT patients. In the present study, bone marrow from beta3-deficient (beta3-/-) mice was transduced with the ITGbeta3-cassette to investigate whether the platelet progeny could establish hemostasis in vivo. A lentivirus transfer vector equipped with the human ITGA2B gene promoter confined transgene expression to the platelet lineage. Human beta3 formed a stable complex with murine alphaIIb, effectively restoring platelet function. Mice expressing significant levels of alphaIIbbeta3 on circulating platelets exhibited improved bleeding times. Intravenous immunoglobulin effectively diminished platelet clearance in animals that developed an antibody response to alphaIIbbeta3. These results indicate the feasibility of targeting platelets with genetic therapies for better management of patients with inherited bleeding disorders.
Figures
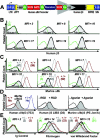
Figure 1.
Expression of a functional, hybrid murine αIIb-human β3 integrin complex. (A) Schematic diagram of lentivirus vector β3-WPTS. The enhancer/promoter of the viral 3′-long terminal repeat (LTR) was removed to allow the vector to self-inactivate (SIN), and the human ITGA2B gene promoter (nucleotides –889 to +35) was used to direct megakaryocyte-specific synthesis of human β3 in mice. The promoter binds GATA and Ets for high-level gene transcription in megakaryocytes, and there is also a repressor region that inhibits gene expression in other lineages. The woodchuck hepatitis virus postregulatory element (WPRE) and the central polypurine tract (cPPT) were used to enhance the efficiency of transgene expression. (B) β3-WPTS–transduced bone marrow was transplanted into β3–/– mice (as described in “Materials and methods”). Flow cytometric histograms of murine platelets isolated from circulating whole blood of transplant recipients A to F showed that 5 mice exhibited significant levels of β3 on their platelet surface (shaded peak) compared with MFI levels for platelets from a β3–/– control (overlay histogram) using a PE-conjugated antibody to human β3. (C) Flow cytometric analysis detected integrin αIIb on the surface of platelets from recipients A to F with an FITC-conjugated antibody to murine αIIb. Histograms showed that mice B to F expressed αIIb on platelets (shaded peak) at intermediate MFI levels compared with the levels (in parentheses) on platelets from β3–/–, β3+/–, and β3+/+ controls. (D, left) Flow cytometric analysis revealed that Alexa 488–conjugated antibody (7E3) specific for human β3 in complex with αIIb or αv reacted positively with platelets from a β3–/– mouse expressing human β3 in complex with murine αIIb (shaded peak) in comparison with murine β3+/+ platelets serving as a negative control and human β3+/+ platelets used as a positive control. (Middle) Platelets from the mouse in the left panel were used to show that a fibrinogen mimetic peptide containing Arg-Gly-Asp (+RGD) could induce murine platelets expressing human β3 (shaded peak) to bind a monoclonal antibody (D3) (plus PE-F(ab′)2 goat anti–murine IgG Fc secondary antibody) that recognizes a ligand induced binding site (LIBS) exposed only on the high-affinity conformation of human β3. The platelets failed to bind D3 in the absence of RGD peptide (–RGD). (Right) Histogram demonstrating that an antibody (PE-Jon/A) specific for the high-affinity conformation of murine αIIbβ3 reacted positively with platelets expressing human β3 from the mouse described in the left and middle panels (shaded peak) following treatment with a cocktail of physiologic agonist of platelet activation (ADP, epinephrine, PAR4). Quiescent platelets were not recognized by PE-Jon/A in the absence of agonist (–Agonist). Results shown were observed in at least 2 separate experiments analyzing platelets from 3 separate mice that expressed human β3 at similar MFI levels. (E) Platelets from the mouse described in panel D were fixed and permeabilized to perform quantitative analysis with rabbit polyclonal antibodies to detect the intracellular storage of major ligands for αIIbβ3, fibrinogen, and VWF. (Left) Histogram shows that a nonreactive Alexa 647 rabbit polyclonal antibody did not react with murine platelets expressing human β3 (shaded peak) nor did it stain platelets from β3–/–, β3+/–, and β3+/+ controls. A nonreactive FITC-Ig showed identical results (not shown). (Middle, right) Histograms reveal that an FITC-antibody to fibrinogen (middle) and an Alexa 647–antibody to VWF (right) recognized platelets from the mouse expressing human β3 (shaded peak) at intermediate MFI levels compared with the level (in parentheses) in platelets from β3–/–, β3+/–, and β3+/+ controls. Results shown were observed in at least 2 separate experiments analyzing platelets from 3 separate mice that expressed human β3 at similar MFI levels.
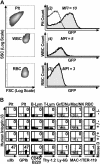
Figure 2.
The human αIIb gene promoter confined transgene expression to platelets. (A) The human αIIb promoter confined expression of a GFP reporter gene within the platelet lineage. Entities exhibiting the forward (FSC) and side (SSC) scattering properties of platelets (Plt), white blood cells (WBC), and red blood cells (RBC) isolated from circulating whole blood of a β3+/+ mouse that received a transplant of –889GFPII-transduced bone marrow (column 1, density plot) were used to construct flow cytometric histograms comparing untransduced and GFP-transduced lineages (column 2). The transplant recipient showed significant levels of GFP in platelets (row 1, column 2, shaded peak) compared with the MFI for platelets from a β3+/+ control that did not receive a bone marrow transplant (open overlay histogram). In contrast, GFP was not detected above background levels within the WBCs or RBCs of the mouse that underwent transplantation. The result shown is representative of the outcome from analysis of peripheral blood collected from one mouse on 5 separate occasions. (B) Two-color flow cytometric analysis showed that the human αIIb promoter targeted expression of human β3 to platelets. A panel of antibodies that react with surface markers (x-axis, bottom) of specific murine cell lineages (x-axis, top) was used in conjunction with an antibody to human β3 (y-axis). The percentage of cells coexpressing both markers is indicated in each density plot (top right quadrant). Human β3 was not detected in cells from β3–/–, β3+/–, and β3+/+ controls (rows 1-3), while mouse D had significant levels of β3 detectable only in platelets (row 4). Plt indicates platelet; B-Lym, B lymphocyte; T-Lym, T lymphocyte; Gr/E/Nu, granulocyte/eosinophil/neutrophil; Mac/NK, macrophage/natural killer cell; and RBC, red blood cell. The result shown is representative of the outcome observed in 4 experiments that analyzed peripheral blood collected from 2 mice on 2 separate occasions.
Figure 3.
Platelet function was restored in recipients of β3-transduced marrow. (A) Aggregation was measured ex vivo following incubation of washed platelets with fibrinogen and a cocktail of activation agonists (ADP, epinephrine, and PAR4). Platelets from β3-transduced marrow recipients A to D as well as β3–/–, β3+/–, and β3+/+ control samples aggregated in direct correlation with the level of β3 on their platelets. These results were observed for each mouse in at least 2 separate experiments. (B) Aggregation was measured ex vivo following a 30-minute pretreatment of platelets at 37°C with αIIbβ3 and αvβ3 complex–specific antibody, 7E3 (known to inhibit platelet aggregation), followed by incubation of washed platelets with human fibrinogen and a cocktail of platelet activation agonist (ADP, epinephrine, and PAR4). Shown is the aggregation profile of a mixture of platelets from β3-transduced marrow recipients E to F, which was increasingly inhibited with higher concentrations of 7E3 (0-50 μg/mL). This result represents the outcome of 3 separate experiments. Aggregation was not inhibited with nonspecific mouse Ig. (C) In vivo platelet function was examined by light microscopic analysis of fixed lung tissue stained with trichrome following intravenous injection of a platelet agonist (ADP) into mice (magnification, 400×). Thromboemboli (blue, arrows) formed in the pulmonary blood vessels (BV) of β3+/– and β3+/+ controls, while platelets in β3–/– animals were unable to form emboli. In contrast to results with β3–/– mice, platelets within transplant recipient C formed emboli that occluded the pulmonary blood vessels. A indicates alveolus; AD, alveolar duct; and TB, terminal bronchiole. This result represents the outcome observed after viewing several sections of each lung from 8 controls (3 β3–/–, 2 β3+/–, and 3 β3+/+ mice) and 3 mice expressing human β3. Images were captured with a Nikon Eclipse TS100 microscope (Nikon, Tokyo, Japan) using a 40×/0.55 numeric aperture objective.
Figure 4.
IVIG treatment diminished an antibody response to αIIbβ3. (A) Flow cytometric histograms showed that plasma from mice A and B contained Ig antibodies (shaded peak) that reacted with normal human platelets. Displayed is the relative fluorescence intensity of human platelets incubated with 1:10 diluted murine plasma and an FITC-conjugated F(ab′)2 goat anti–murine IgG Fc secondary antibody. Platelets incubated with secondary antibody and dilution buffer served as a negative control, while a monoclonal antibody to the human αIIbβ3 complex (AP2) was used as a positive control. This result was observed 4 times using platelets from 2 separate human donors analyzed on 2 separate occasions. (B) As in panel A, fluorescence analysis showed that 1:10 diluted plasma from mouse A also reacted with platelets from normal β3+/+, heterozygous β3+/– mice as well as platelets from another human β3-transduced transplant recipient (shaded peak). In striking contrast, plasma did not react with platelets isolated from a β3–/– mouse. Dilution buffer served as the negative control and a PE-conjugated monoclonal antibody to murine GPIbα was used as a positive control. (C) IVIG (0.5 mg/g body weight) was injected each day for 3 days into mouse A. Flow cytometry was then performed with human platelets incubated in plasma from mouse A and secondary antibody as described in panel A. The level of plasma Ig binding to human platelets before and after IVIG treatment of mouse A (black line) was determined by dividing the MFI of platelets incubated with mouse A plasma by the MFI of platelets treated with negative control buffer. The ratio decreased below 2.0 (dotted line), indicating a negligible affinity of plasma Ig for platelet proteins. The overlay graph shows an increase in platelets expressing αIIbβ3 following IVIG treatment of mouse A (orange line). The MFI ratio was calculated from flow-cytometric histograms detecting the binding of an FITC-conjugated antibody against murine αIIb to platelets from mouse A versus antibody binding to β3–/– platelets. These results represent the outcome of IVIG treatment for 3 mice with detectable plasma Ig to human platelets. (D) Following IVIG treatment, mouse A had restored platelet function in an aggregation assay performed at 27 weeks after transplantation. This result was observed using platelets from mouse A and platelets isolated from mice that received a transplant of bone marrow derived from mouse A as second- and third-generation recipients. (E) As in panel C, flow cytometric analysis using the MFI ratio of platelets binding an FITC-conjugated antibody to murine αIIb demonstrated long-term (32 weeks), stable expression of αIIbβ3 on the surface of platelets from mouse A after IVIG and mice B and D. Note: mouse C was killed for the in vivo platelet function assay at week 5.
Similar articles
- A novel Pro126His beta propeller mutation in integrin alphaIIb causes Glanzmann thrombasthenia by impairing progression of pro-alphaIIbbeta3 from endoplasmic reticulum to Golgi.
Shen WZ, Ding QL, Jin PP, Wang XF, Jiang YZ, Li SM, Wang HL. Shen WZ, et al. Blood Cells Mol Dis. 2009 Jan-Feb;42(1):44-50. doi: 10.1016/j.bcmd.2008.08.005. Epub 2008 Oct 30. Blood Cells Mol Dis. 2009. PMID: 18976939 - Platelet gene therapy improves hemostatic function for integrin alphaIIbbeta3-deficient dogs.
Fang J, Jensen ES, Boudreaux MK, Du LM, Hawkins TB, Koukouritaki SB, Cornetta K, Wilcox DA. Fang J, et al. Proc Natl Acad Sci U S A. 2011 Jun 7;108(23):9583-8. doi: 10.1073/pnas.1016394108. Epub 2011 May 23. Proc Natl Acad Sci U S A. 2011. PMID: 21606353 Free PMC article. - In silico analysis of structural modifications in and around the integrin αIIb genu caused by ITGA2B variants in human platelets with emphasis on Glanzmann thrombasthenia.
Pillois X, Peters P, Segers K, Nurden AT. Pillois X, et al. Mol Genet Genomic Med. 2018 Mar;6(2):249-260. doi: 10.1002/mgg3.365. Epub 2018 Jan 31. Mol Genet Genomic Med. 2018. PMID: 29385657 Free PMC article. - Glanzmann thrombasthenia: state of the art and future directions.
Nurden AT, Pillois X, Wilcox DA. Nurden AT, et al. Semin Thromb Hemost. 2013 Sep;39(6):642-55. doi: 10.1055/s-0033-1353393. Epub 2013 Aug 8. Semin Thromb Hemost. 2013. PMID: 23929305 Free PMC article. Review. - Glanzmann thrombasthenia-like syndromes associated with Macrothrombocytopenias and mutations in the genes encoding the αIIbβ3 integrin.
Nurden AT, Pillois X, Fiore M, Heilig R, Nurden P. Nurden AT, et al. Semin Thromb Hemost. 2011 Sep;37(6):698-706. doi: 10.1055/s-0031-1291380. Epub 2011 Nov 18. Semin Thromb Hemost. 2011. PMID: 22102273 Review.
Cited by
- Clinical and Economic Impact of a Multidisciplinary Follow-Up Program in Lymphoma Patients.
Devaux M, Boulin M, Mounier M, Caillot D, Ahwij N, Herbin A, Bastie JN, Favennec C, Robert P, Pistre P, Bost S, Amiot P, Jacquesson L, Casasnovas O, Rossi C, Gueneau P. Devaux M, et al. Cancers (Basel). 2022 May 21;14(10):2532. doi: 10.3390/cancers14102532. Cancers (Basel). 2022. PMID: 35626136 Free PMC article. - Inherited Platelet Disorders: An Updated Overview.
Palma-Barqueros V, Revilla N, Sánchez A, Zamora Cánovas A, Rodriguez-Alén A, Marín-Quílez A, González-Porras JR, Vicente V, Lozano ML, Bastida JM, Rivera J. Palma-Barqueros V, et al. Int J Mol Sci. 2021 Apr 26;22(9):4521. doi: 10.3390/ijms22094521. Int J Mol Sci. 2021. PMID: 33926054 Free PMC article. Review. - C560Rβ3 caused platelet integrin αII b β3 to bind fibrinogen continuously, but resulted in a severe bleeding syndrome and increased murine mortality.
Fang J, Nurden P, North P, Nurden AT, Du LM, Valentin N, Wilcox DA. Fang J, et al. J Thromb Haemost. 2013 Jun;11(6):1163-71. doi: 10.1111/jth.12209. J Thromb Haemost. 2013. PMID: 23551977 Free PMC article. - Concordance of preclinical and clinical pharmacology and toxicology of therapeutic monoclonal antibodies and fusion proteins: cell surface targets.
Bugelski PJ, Martin PL. Bugelski PJ, et al. Br J Pharmacol. 2012 Jun;166(3):823-46. doi: 10.1111/j.1476-5381.2011.01811.x. Br J Pharmacol. 2012. PMID: 22168282 Free PMC article. Review. - [The progresses in research and treatment of inherited platelet disorders].
Wang ZY, Ruan CG. Wang ZY, et al. Zhonghua Xue Ye Xue Za Zhi. 2018 Oct 14;39(10):877-880. doi: 10.3760/cma.j.issn.0253-2727.2018.10.019. Zhonghua Xue Ye Xue Za Zhi. 2018. PMID: 30369214 Free PMC article. Chinese. No abstract available.
References
- Hynes RO, Zhao Q. The evolution of cell adhesion. J Cell Biol. 2000;150: F89-F96. - PubMed
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110: 673-687. - PubMed
- Wagner CL, Mascelli MA, Neblock DS, Weisman HF, Coller BS, Jordan RE. Analysis of GPIIb/IIIa receptor number by quantification of 7E3 binding to human platelets. Blood. 1996;88: 907-914. - PubMed
- Prandini MH, Martin F, Thevenon D, Uzan G. The tissue-specific transcriptional regulation of the megakaryocytic glycoprotein IIb gene is controlled by interactions between a repressor and positive cis-acting elements. Blood. 1996;88: 2062-2070. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous