Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway - PubMed (original) (raw)
Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway
Zhe-Yu Chen et al. J Neurosci. 2005.
Abstract
Brain-derived neurotrophic factor (BDNF), after activity-dependent secretion from neurons, modulates critical nervous system functions. Recently, a variant in the human bdnf gene, resulting in a valine to methionine substitution in the prodomain, has been shown to lead to defective regulated secretion from neurons and memory impairment. Here, we report a novel function for a Vps10p domain protein, sortilin, in controlling BDNF sorting to the regulated secretory pathway. Sortilin interacts specifically with BDNF in a region encompassing the methionine substitution and colocalizes with BDNF in secretory granules in neurons. A truncated form of sortilin causes BDNF missorting to the constitutive secretory pathway without affecting neurotrophin-4 (NT-4) secretion. In addition, sortilin small interfering RNA introduced into primary neurons also led to BDNF missorting from the regulated to the constitutive secretory pathway. Together, these data suggest a mechanism to understand the defect associated with variant BDNF and provide a framework, based on divergent presynaptic regulation of sorting to secretory pathways, to explain how two ligands for tropomyosin-related kinase B, BDNF and NT-4, can mediate diverse biological responses.
Figures
Figure 1.
Coimmunoprecipitation of sortilin with wild-type and variant BDNF. A, HEK 293 cells were cotransfected with cDNAs encoding sortilin and C-terminal BDNFVal-HA or BDNFMet-HA or empty vector (-). Cell lysates were immunoprecipitated with HA antibodies and immunoblotted with sortilin antibodies (top). Immunoprecipitation (IP) of BDNF-HA was confirmed by immunoblotting with HA antibodies (middle). Crude lysates were immunoblotted with sortilin antibodies and HA antibodies to confirm equivalent expression level (bottom). B, In vitro binding assay. GST-BDNFVal, GST-BDNFMet, or GST-NT-4 prodomain fusion proteins were incubated with lysates from HEK 293 cells overexpressing full-length sortilin. Bound sortilin was detected by immunoblotting with anti-sortilin antibody.
Figure 2.
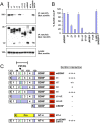
Mapping the interaction of sortilin with BDNF mutants. A, HEK 293 cells were cotransfected with sortilin and either full-length BDNF or NT-4 or truncation mutants. Lysates were immunoprecipitated with HA antibodies and immunoblotted with sortilin antibodies (top). Immunoprecipitation of BDNF-HA was confirmed by immunoblotting with HA antibodies (middle). Sortilin expression was verified with sortilin antibodies (bottom). B, Quantitation of Western blots in the top panel of A as a percentage of sortilin coimmunoprecipitation (Co-IP) by mutant neurotrophins compared with wild-type BDNF. Mean proportions ± SEM were determined from analysis of three independent experiments. C, Summary of BDNF and NT-4 mutants used in A and a summary of the sortilin interactions with the mutant BDNF and NT-4. IP, Immunoprecipitation; wt, wild type; m, mature.
Figure 3.
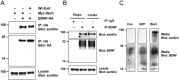
Mapping the interaction of BDNF with sortilin mutants. A, Coimmunoprecipitation of tSort with BDNF. HEK 293 cells were cotransfected with cDNAs encoding a mutant truncated sortilin or full-length sortilin along with C-terminal-tagged BDNF-HA. Cell lysates were immunoprecipitated with HA antibodies and immunoblotted with sortilin antibodies (top). Immunoprecipitation of BDNF-HA was confirmed by immunoblotting with HA antibodies (middle). Crude lysates were immunoblotted with sortilin antibodies to confirm equivalent expression level (bottom). B, Endogenous association of BDNF with sortilin. P10 hippocampal and cortical lysates were subjected to immunoprecipitation with anti-BDNF antibodies or normal rabbit IgG and immunoblotted with sortilin antibodies (top). Immunoprecipitation of BDNF was confirmed by immunoblotting with BDNF antibodies (middle). Crude lysates were immunoblotted with sortilin antibodies to confirm equivalent protein loading (bottom). C, Constitutive secretion of BDNF in the absence and presence of truncated sortilin. HEK 293 cells stably expressing proBDNF were infected with recombinant adenovirus encoding GFP or tSort at multiplicity of infection of 1. Media (25 μg per lane) was then collected after 48 h after infection and immunoblotted with BDNF and sortilin antibodies. Wt, Wild type; IP, immunoprecipitation; Hippo, hippocampus; Con, control.
Figure 4.
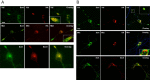
Subcellular colocalization of variant and wild-type BDNF with sortilin. A, Differentiated PC12 cells were transfected with N-terminal HA-tagged variant (Met) or wild-type BDNF (Val). Cells were fixed after 48 h and permeabilized. Colocalization of BDNF and sortilin was visualized by costaining with anti-HA and anti-sortilin antibodies. In addition, untransfected differentiated PC12 cells were fixed after 48 h and permeabilized. Colocalization of endogenous sortilin and SecII was visualized by costaining with anti-sortilin and anti-SecII antibodies. Representative epifluorescence images are shown. B, Hippocampal neurons were transfected with N-terminal HA-tagged variant (Met) or wild-type BDNF (Val) and Myc-tagged sortilin. Cells were fixed after 48 h and permeabilized. Colocalization of HA-BDNF and Myc-sortilin was visualized by confocal microscopy, as described in Materials and Methods, using epitope antibodies. All neurons were confirmed using MAP2 antibodies. In addition, untransfected neurons were fixed after 48 h and permeabilized. Colocalization of endogenous sortilin and SecII was visualized by costaining with anti-sortilin and anti-SecII antibodies. Representative epifluorescence images are shown. Scale bars, 20 μm.
Figure 5.
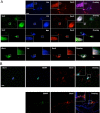
Truncated sortilin alters intracellular BDNF targeting to secretory granules. A, Differentiated PC12 cells were cotransfected with full-length or truncated Myc-tagged sortilin along with N-terminal HA-tagged BDNFVal or BDNFMet. Cells were fixed after 48 h and permeabilized. Localization of sortilin (full-length, truncated), BDNF, and SecII was visualized by costaining with anti-HA, anti-Myc antibodies, and anti-SecII antibodies. Representative epifluorescence images are shown. B, Hippocampal neurons were cotransfected with truncated Myc-tagged sortilin along with N-terminal HA-tagged BDNF. Cells were fixed after 48 h and permeabilized. Localization of BDNF and SecII was visualized by costaining with anti-HA, anti-SecII antibodies, and anti-MAP2 antibodies. Localization of truncated sortilin and BDNF and SecII was visualized by costaining with anti-HA, anti-Myc antibodies, and anti-SecII antibodies. Representative epifluorescence images are shown. Scale bars, 20 μm.
Figure 6.
Role of the BDNF prodomain on activity-dependent BDNF secretion. A, Lentiviral constructs containing differential regions of the BDNF prodomain (Box1, Box2, Box3, Box2+ 3) fused to GFP or GFP alone were expressed in PC12 cells transfected with N-terminal HA-BDNF. After 48 h, media were collected under depolarization secretion conditions, as described in Materials and Methods, and analyzed by ELISA. Results are presented as a mean ± SEM determined from six independent experiments (**p < 0.001; Student's t test). B, Differentiated PC12 cells were transfected with mutant forms of BDNF lacking certain regions of the prodomain (ΔBox1, ΔBox2′, ΔBox3, ΔBox2 + 3). After 48 h, media were collected under depolarization secretion conditions, as described in Materials and Methods, and analyzed by ELISA. Results are presented as a mean ± SEM determined from analysis of six independent experiments (**p < 0.001; Student's t test). WT, Wild type.
Figure 7.
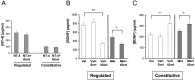
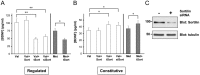
Truncated sortilin selectively alters activity-dependent BDNF secretion. A, Differentiated PC12 cells were transfected with NT-4 in the presence or absence of tSort. After 48 h, media were collected under depolarization and constitutive secretion conditions, as described in Materials and Methods, and analyzed by ELISA. Differentiated PC12 cells were transfected with BDNF in the presence or absence of full-length sortilin (Sort) or tSort. After 48 h, media were collected under depolarization (B) and constitutive secretion (C) conditions, as described in Materials and Methods, and analyzed by ELISA. All results are presented as a mean ± SEM determined from analysis of six independent experiments (*p < 0.01; **p < 0.001; Student's t test).
Figure 8.
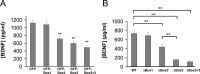
Truncated sortilin and sortilin siRNA selectively alter activity-dependent BDNF secretion. Hippocampo-cortical neurons were transfected with BDNF in the presence or absence of full-length sortilin (Sort), tSort, or sortilin siRNA (siSort). After 3 d, media were collected under depolarization (A) and constitutive secretion (B) conditions, as described in Materials and Methods, and analyzed by ELISA. All results are presented as a mean ± SEM determined from analysis of six independent experiments (*p < 0.05; **p < 0.01; Student's t test). C, Hippocampo-cortical neurons were transfected in the presence or absence of sortilin siRNA. After 3 d, cells lysates were immunoblotted for and sortilin and tubulin (loading control) as described in Materials and Methods.
Similar articles
- Role of endogenous brain-derived neurotrophic factor and sortilin in B cell survival.
Fauchais AL, Lalloué F, Lise MC, Boumediene A, Preud'homme JL, Vidal E, Jauberteau MO. Fauchais AL, et al. J Immunol. 2008 Sep 1;181(5):3027-38. doi: 10.4049/jimmunol.181.5.3027. J Immunol. 2008. PMID: 18713973 - Neuronal brain-derived neurotrophic factor is synthesized in excess, with levels regulated by sortilin-mediated trafficking and lysosomal degradation.
Evans SF, Irmady K, Ostrow K, Kim T, Nykjaer A, Saftig P, Blobel C, Hempstead BL. Evans SF, et al. J Biol Chem. 2011 Aug 26;286(34):29556-67. doi: 10.1074/jbc.M111.219675. Epub 2011 Jul 5. J Biol Chem. 2011. PMID: 21730062 Free PMC article. - ProBDNF induces neuronal apoptosis via activation of a receptor complex of p75NTR and sortilin.
Teng HK, Teng KK, Lee R, Wright S, Tevar S, Almeida RD, Kermani P, Torkin R, Chen ZY, Lee FS, Kraemer RT, Nykjaer A, Hempstead BL. Teng HK, et al. J Neurosci. 2005 Jun 1;25(22):5455-63. doi: 10.1523/JNEUROSCI.5123-04.2005. J Neurosci. 2005. PMID: 15930396 Free PMC article. - Brain-Derived Neurotrophic Factor in Suicide Pathophysiology.
Dwivedi Y. Dwivedi Y. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton (FL): CRC Press/Taylor & Francis; 2012. Chapter 8. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton (FL): CRC Press/Taylor & Francis; 2012. Chapter 8. PMID: 23035300 Free Books & Documents. Review. - Multi-tasking by the p75 neurotrophin receptor: sortilin things out?
Bronfman FC, Fainzilber M. Bronfman FC, et al. EMBO Rep. 2004 Sep;5(9):867-71. doi: 10.1038/sj.embor.7400219. EMBO Rep. 2004. PMID: 15470383 Free PMC article. Review.
Cited by
- Molecular and genetic basis of depression.
Roy M, Tapadia MG, Joshi S, Koch B. Roy M, et al. J Genet. 2014 Dec;93(3):879-92. doi: 10.1007/s12041-014-0449-x. J Genet. 2014. PMID: 25572252 Review. - Fear extinction and BDNF: translating animal models of PTSD to the clinic.
Andero R, Ressler KJ. Andero R, et al. Genes Brain Behav. 2012 Jul;11(5):503-12. doi: 10.1111/j.1601-183X.2012.00801.x. Epub 2012 May 11. Genes Brain Behav. 2012. PMID: 22530815 Free PMC article. Review. - Sequence specificity despite intrinsic disorder: How a disease-associated Val/Met polymorphism rearranges tertiary interactions in a long disordered protein.
Lohia R, Salari R, Brannigan G. Lohia R, et al. PLoS Comput Biol. 2019 Oct 18;15(10):e1007390. doi: 10.1371/journal.pcbi.1007390. eCollection 2019 Oct. PLoS Comput Biol. 2019. PMID: 31626641 Free PMC article. - The Peptide-Drug Conjugate TH1902: A New Sortilin Receptor-Mediated Cancer Therapeutic against Ovarian and Endometrial Cancers.
Currie JC, Demeule M, Charfi C, Zgheib A, Larocque A, Danalache BA, Ouanouki A, Béliveau R, Marsolais C, Annabi B. Currie JC, et al. Cancers (Basel). 2022 Apr 8;14(8):1877. doi: 10.3390/cancers14081877. Cancers (Basel). 2022. PMID: 35454785 Free PMC article. - Recent advances in understanding context-dependent mechanisms controlling neurotrophin signaling and function.
Bothwell M. Bothwell M. F1000Res. 2019 Sep 19;8:F1000 Faculty Rev-1658. doi: 10.12688/f1000research.19174.1. eCollection 2019. F1000Res. 2019. PMID: 31583078 Free PMC article. Review.
References
- Berg EA, Johnson RJ, Leeman SE, Boyd N, Kimerer L, Fine RE (2000) Isolation and characterization of substance P-containing dense core vesicles from rabbit optic nerve and termini. J Neurosci Res 62: 830-839. - PubMed
- Berkemeier LR, Winslow JW, Kaplan DR, Nikolics K, Goeddel DV, Rosenthal A (1991) Neurotrophin-5: a novel neurotrophic factor that activates trk and trkB. Neuron 7: 857-866. - PubMed
- Chao MV (2003) Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci 4: 299-309. - PubMed
- Chen ZY, Patel PD, Sant G, Meng CX, Teng KK, Hempstead BL, Lee FS (2004) Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci 24: 4401-4411. - PMC - PubMed
- Egan M, Kojima M, Callicott J, Goldberg T, Kolachana B, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger D (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112: 257-269. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials