Altered axonal architecture by removal of the heavily phosphorylated neurofilament tail domains strongly slows superoxide dismutase 1 mutant-mediated ALS - PubMed (original) (raw)
Comparative Study
. 2005 Jul 19;102(29):10351-6.
doi: 10.1073/pnas.0503862102. Epub 2005 Jul 7.
Affiliations
- PMID: 16002469
- PMCID: PMC1177385
- DOI: 10.1073/pnas.0503862102
Comparative Study
Altered axonal architecture by removal of the heavily phosphorylated neurofilament tail domains strongly slows superoxide dismutase 1 mutant-mediated ALS
Christian S Lobsiger et al. Proc Natl Acad Sci U S A. 2005.
Abstract
Eliminating assembled neurofilaments (NFs) from axons or misaccumulating NFs in motor neuron cell bodies strongly slows disease in mouse models of mutant superoxide dismutase 1 (SOD1)-induced amyotrophic lateral sclerosis. One proposal for how reducing axonal NFs can increase survival is that the multiphosphorylated tail domains of the two larger NF subunits act in motor neuron cell bodies as phosphorylation sinks where they mitigate cyclin-dependent kinase 5 dysregulation induced by mutant SOD1. Elimination by gene targeting in mice of the NF medium and NF heavy tail domains and their 58 known phosphorylation sites accelerates aberrant phosphorylation of other neuronal substrates while leaving overall NF content unaltered. However, disease onset is significantly delayed and survival is extended, inconsistent with the ameliorative property of altered NF content protecting by serving as substrates for dysregulation of any NF kinase. Moreover, at comparable disease stages significantly more surviving motor neurons and axons were found in SOD1 mutant mice deleted in the NF tails than in similar mice with wild-type NFs. This finding supports noncell autonomous toxicity in SOD1 mutant-mediated amyotrophic lateral sclerosis: removal of the NF tails slows damage developed directly within motor neurons, but SOD1 mutant damage within nonneuronal supporting cells reduces motor neuron functionality.
Figures
Fig. 1.
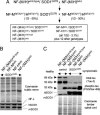
Generation and immunoblot analysis of mutant SOD1 mice lacking the NF tail domains. (A) Mating scheme to obtain the six genotypes followed for this study (boxed) consisting of mutant SOD1 mice (SOD1G37R) with either normal NF content [NF-(M/H)wild-type/SOD1G37R] or that are deleted in one or both of the NF tail domains [NF-MtailΔ/SOD1G37R, NF-HtailΔ/SOD1G37R, and NF-(M/H)tailΔ/SOD1G37R], plus the two control genotypes without mutant SOD1 (expected Mendelian frequencies are indicated). (B) Parallel immunoblots of sciatic nerve extracts from the four mutant SOD1 mice containing normal or tail domain-deleted NFs, showing unchanged levels of NFs (as determined by NF-L) and of both mouse and mutant human SOD1 proteins (m/hSOD1). (C) Parallel immunoblots of spinal cord extracts confirming that the NF-M/H tail domains can act as phosphorylation sinks against Cdk5 targets like tau (see text). Control animals were 6 months old, whereas mutant SOD1 mice were at hind limb weakness. Equal loading is shown by Coomassie and the endogenous mouse SOD1 (mSOD1), whereas total tau was detected with tau-5 and phospho-tau with PG5 antibodies. Note that because overall total tau levels are down-regulated in mutant SOD1 mice (see text) the ratio of phosho/unphospho-tau is actually higher in the NF-(M/H)wild-type/SOD1G37R mice than in the control NF-(M/H)tailΔ mice, although the total phospho-tau level is reduced, consistent with a mutant SOD1-induced deregulation of Cdk5 (24).
Fig. 2.
Removing the NF tail domains delayed disease onset and extended survival in mutant SOD1 mice. Kaplan-Meier curves showing age (and delay Δ in months) of disease onset (weight peak) (A), hind limb weakness (10% weight loss) (B), and end stage (hind limb paralysis) (C) of mutant SOD1 mice with normal or tail domain-deleted NF content. The respective average ages (with SEM and number of animals) are indicated on the right including the mutant SOD1 mice that had only one NF tail domain deleted.
Fig. 3.
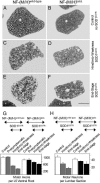
Mutant SOD1-induced loss of motor neurons is reduced in mice lacking both NF tail domains despite similar disease stages. (A_–_F) L5 ventral root axons of control mice without mutant SOD1 with no obvious axonal degeneration at 14 months of age (A and B) and mutant SOD1 mice with normal (C and E) or tail domain-deleted (D and F) NF content both at hind limb weakness (C and D) and end stage (E and F). (Scale bar: 200 μm, F.) (G and H) Quantitative analysis of the loss of L5 ventral root axons (G) and total L3-L6 lumbar ventral horn motor neuron cell bodies (H) [error bars are SEM; n = 3–4 animals for axon and neuron counts, except for neurons in end stage NF-(M/H)tailΔ/SOD1G37R mice where n = 2]. Mice were analyzed before onset (presymptomatic, at 6 months), at hind limb weakness, and at end stage. Control mice without mutant SOD1 were analyzed at 14 months.
Fig. 4.
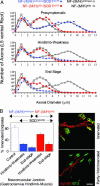
Mutant SOD1 mice lacking both NF tail domains showed a slowing of axonal degeneration and reduced muscle denervation. (A) L5 ventral root axon caliber distributions before onset (presymptomatic, at 6 months), at hind limb weakness, and at end stage with normal or tail domain-deleted NF content and with or without mutant SOD1. Points represent the averaged distributions of diameters from three to four animals. Control mice without mutant SOD1 were analyzed at 14 months of age. (B) Determination of the percentage of innervated endplates in the gastrocnemius hind limb muscle. (Left) Quantitative analysis of denervation in mutant SOD1 mice with or without NF tail domains and in 14-month-old control animals without mutant SOD1. Shown are averages with SEM (n = 3 animals; ≈150 bungarotoxin-positive end plates per animal were randomly chosen and analyzed). (Right) α-Bungarotoxin (BTX, green) was used to identify the postsynaptic domain, whereas NF (red) and synaptophysin (Syn, red) were used to identify axons and presynaptic terminals. (Upper) Yellow area of overlap identifies innervated end plates, while (Lower) partly innervated or completely denervated end plates show only green α-bungarotoxin staining. (Scale bar: 50 μm.)
Similar articles
- Absence of neurofilaments reduces the selective vulnerability of motor neurons and slows disease caused by a familial amyotrophic lateral sclerosis-linked superoxide dismutase 1 mutant.
Williamson TL, Bruijn LI, Zhu Q, Anderson KL, Anderson SD, Julien JP, Cleveland DW. Williamson TL, et al. Proc Natl Acad Sci U S A. 1998 Aug 4;95(16):9631-6. doi: 10.1073/pnas.95.16.9631. Proc Natl Acad Sci U S A. 1998. PMID: 9689132 Free PMC article. - Extra axonal neurofilaments do not exacerbate disease caused by mutant Cu,Zn superoxide dismutase.
Couillard-Després S, Meier J, Julien JP. Couillard-Després S, et al. Neurobiol Dis. 2000 Aug;7(4):462-70. doi: 10.1006/nbdi.2000.0296. Neurobiol Dis. 2000. PMID: 10964615 - Human Cu/Zn superoxide dismutase (SOD1) overexpression in mice causes mitochondrial vacuolization, axonal degeneration, and premature motoneuron death and accelerates motoneuron disease in mice expressing a familial amyotrophic lateral sclerosis mutant SOD1.
Jaarsma D, Haasdijk ED, Grashorn JA, Hawkins R, van Duijn W, Verspaget HW, London J, Holstege JC. Jaarsma D, et al. Neurobiol Dis. 2000 Dec;7(6 Pt B):623-43. doi: 10.1006/nbdi.2000.0299. Neurobiol Dis. 2000. PMID: 11114261 - Transgenic mice in the study of ALS: the role of neurofilaments.
Julien JP, Couillard-Després S, Meier J. Julien JP, et al. Brain Pathol. 1998 Oct;8(4):759-69. doi: 10.1111/j.1750-3639.1998.tb00199.x. Brain Pathol. 1998. PMID: 9804382 Free PMC article. Review. - Defective neurofilament transport in mouse models of amyotrophic lateral sclerosis: a review.
Rao MV, Nixon RA. Rao MV, et al. Neurochem Res. 2003 Jul;28(7):1041-7. doi: 10.1023/a:1023259207015. Neurochem Res. 2003. PMID: 12737529 Review.
Cited by
- Mutant SOD1 in cell types other than motor neurons and oligodendrocytes accelerates onset of disease in ALS mice.
Yamanaka K, Boillee S, Roberts EA, Garcia ML, McAlonis-Downes M, Mikse OR, Cleveland DW, Goldstein LS. Yamanaka K, et al. Proc Natl Acad Sci U S A. 2008 May 27;105(21):7594-9. doi: 10.1073/pnas.0802556105. Epub 2008 May 20. Proc Natl Acad Sci U S A. 2008. PMID: 18492803 Free PMC article. - Inhibition of Crmp1 Phosphorylation at Ser522 Ameliorates Motor Function and Neuronal Pathology in Amyotrophic Lateral Sclerosis Model Mice.
Asano T, Nakamura H, Kawamoto Y, Tada M, Kimura Y, Takano H, Yao R, Saito H, Ikeda T, Komiya H, Kubota S, Hashiguchi S, Takahashi K, Kunii M, Tanaka K, Goshima Y, Nakamura F, Takeuchi H, Doi H, Tanaka F. Asano T, et al. eNeuro. 2022 May 23;9(3):ENEURO.0133-22.2022. doi: 10.1523/ENEURO.0133-22.2022. Print 2022 May-Jun. eNeuro. 2022. PMID: 35523582 Free PMC article. - A Systematic Review of Suggested Molecular Strata, Biomarkers and Their Tissue Sources in ALS.
Vijayakumar UG, Milla V, Cynthia Stafford MY, Bjourson AJ, Duddy W, Duguez SM. Vijayakumar UG, et al. Front Neurol. 2019 May 14;10:400. doi: 10.3389/fneur.2019.00400. eCollection 2019. Front Neurol. 2019. PMID: 31139131 Free PMC article. Review. - A disease- and phosphorylation-related nonmechanical function for keratin 8.
Ku NO, Omary MB. Ku NO, et al. J Cell Biol. 2006 Jul 3;174(1):115-25. doi: 10.1083/jcb.200602146. J Cell Biol. 2006. PMID: 16818723 Free PMC article.
References
- Mulder, D. W., Kurland, L. T., Offord, K. P. & Beard, C. M. (1986) Neurology 36, 511-517. - PubMed
- Rosen, D. R., Siddique, T., Patterson, D., Figlewicz, D. A., Sapp, P., Hentati, A., Donaldson, D., Goto, J., O'Regan, J. P., Deng, H. X., et al. (1993) Nature 362, 59-62. - PubMed
- Bruijn, L. I., Miller, T. M. & Cleveland, D. W. (2004) Annu. Rev. Neurosci. 27, 723-749. - PubMed
- Gurney, M. E., Pu, H., Chiu, A. Y., Dal Canto, M. C., Polchow, C. Y., Alexander, D. D., Caliendo, J., Hentati, A., Kwon, Y. W., Deng, H. X., et al. (1994) Science 264, 1772-1775. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous