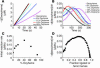Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control - PubMed (original) (raw)
. 2005 Aug;115(8):2099-107.
doi: 10.1172/JCI24650. Epub 2005 Jul 21.
Affiliations
- PMID: 16041407
- PMCID: PMC1177999
- DOI: 10.1172/JCI24650
Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control
Zhi Huang et al. J Clin Invest. 2005 Aug.
Abstract
Hypoxic vasodilation is a fundamental, highly conserved physiological response that requires oxygen and/or pH sensing coupled to vasodilation. While this process was first characterized more than 80 years ago, the precise identity and mechanism of the oxygen sensor and mediators of vasodilation remain uncertain. In support of a possible role for hemoglobin (Hb) as a sensor and effector of hypoxic vasodilation, here we show biochemical evidence that Hb exhibits enzymatic behavior as a nitrite reductase, with maximal NO generation rates occurring near the oxy-to-deoxy (R-to-T) allosteric structural transition of the protein. The observed rate of nitrite reduction by Hb deviates from second-order kinetics, and sigmoidal reaction progress is determined by a balance between 2 opposing chemistries of the heme in the R (oxygenated conformation) and T (deoxygenated conformation) allosteric quaternary structures of the Hb tetramer--the greater reductive potential of deoxyheme in the R state tetramer and the number of unligated deoxyheme sites necessary for nitrite binding, which are more plentiful in the T state tetramer. These opposing chemistries result in a maximal nitrite reduction rate when Hb is 40-60% saturated with oxygen (near the Hb P50), an apparent ideal set point for hypoxia-responsive NO generation. These data suggest that the oxygen sensor for hypoxic vasodilation is determined by Hb oxygen saturation and quaternary structure and that the nitrite reductase activity of Hb generates NO gas under allosteric and pH control.
Figures
Figure 1
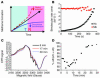
Reaction of nitrite with deoxyHb reveals deviation from first-order kinetics and equimolar product yields of metHb and iron-nitrosyl-Hb. (A) Progress of the nitrite-Hb reaction under conditions of excess nitrite (10 mM nitrite with 50 μM heme) monitored spectrophotometrically by formation of ferric hemes at 630 nm (baseline at 700 nm) under anaerobic conditions or during partial oxygenation (18% oxyHb). 50 μM metHb gives A630 of 0.12 with 10 mM nitrite present. (B) The fit of the natural log of deoxyheme concentration versus time for conditions shown in A, where _Ln_[deoxyheme] is the natural log of the deoxyheme concentration at each time point. The deoxyheme concentration at each time point was determined by spectral deconvolution. (C) Effects of oxygen leak on product yields in the nitrite-Hb reaction (calculated by spectral deconvolution for reactions in A). (D) Instantaneous rate of deoxyheme consumption over time during the course of a single reaction under the conditions in A. The instantaneous rate of deoxyheme consumption was found by negative change of deoxyheme concentration between 2 adjacent time points (–_d_[deoxyheme]) over the time interval (dt) where d is change and t is time. (E) Progress of the reaction of nitrite (10 mM) with varying initial concentrations of deoxyheme monitored by the formation of metheme at 630 nm. (F) Effect of varying the initial deoxyheme concentration (concentration of deoxyheme at the beginning of the reaction; [DeoxyHb]initial) on the initial rate of the reaction calculated from the data in E. The initial reaction rate is the rate of ferric heme formation at the beginning of the reaction calculated as the average rate over the first 200 seconds using extinction coefficient of 3.4 at 630 nm (the rate of deoxyheme consumption is approximately twice as fast).
Figure 2
Sigmoidal reaction behavior of the nitrite-deoxyheme reaction occurs due to T-to-R allosteric quaternary transition of Hb. (A) Model of the nitrite-deoxyHb reaction representing a balance between 2 opposing processes: (a) a decelerating reaction with T state deoxyheme due to depletion of deoxyheme available for reaction with nitrite because of conversion to metheme and iron-nitrosyl-heme (solid blue line); and (b) an accelerating reaction of nitrite with R state deoxyheme (dashed line). Solid black line with arrow represents the reaction process observed experimentally and is the balance of these 2 processes. (B) Apparent bimolecular rate constant over the time course of the anaerobic reaction of Mb (50 μM heme) and Hb (50 μM heme) with nitrite (10 mM with Hb and 2.5 mM with Mb in heme concentrations). Bimolecular rate constant (const.) was obtained by dividing the instantaneous reaction rate of deoxyheme consumption by the concentration of deoxyheme and nitrite. (C) Initial (6 minutes), intermediate (17 minutes), and final (30 minutes) EPR spectra of iron-nitrosyl-heme monitored over the course of the nitrite-deoxyHb reaction (100 μM heme, 2.5 mM nitrite) showing a transition from 5-coordinate (T state) α iron-nitrosyl-heme (with characteristic hyperfine splitting) to 6-coordinate (R state) heme geometry. The smaller EPR signals at earlier time points were normalized to that of the final time point in order to compare the spectral shape of the iron-nitrosyl-Hb signal. (D) T-to-R allosteric structural transition during the course of the nitrite-deoxyHb reaction, monitored by the percentage formation of 6-coordinate α iron-nitrosyl-heme relative to total α iron-nitrosyl-heme.
Figure 3
Rate of nitrite reductase reaction and NO gas formation is under allosteric control. (A) Progress of the anaerobic reaction of Mb (50 μM heme) and Hb (50 μM heme) with nitrite (10 mM with Hb and 2.5 mM with Mb in heme concentrations) monitored spectrophotometrically by metHb formation at 630 nm. (B) First order fits for Mb and non–first order behavior of tetrameric Hb (fits of natural log of deoxyheme concentration for the same reactions shown in A). (C) Simultaneous measurement of NO gas by chemiluminescence during the course of the reaction shown in A. Inset shows the instantaneous rate of deoxyheme consumption over the course of the reaction, obtained from spectral deconvolution. (D) The time to peak NO production measured by chemiluminescence for the reaction of nitrite (10 mM) with Hb (50 μM heme) with varying saturation (0–75%) of carbon monoxide. %HbCO, percentage of Hb that is saturated with carbon monoxide. (E) Reaction progress for β chains of HbA (locked in R state tetramer; 35 μM heme reacted with 0.5 mM nitrite at pH 7.0) and IHP-treated Hb (locked into T state tetramer; 50 μM heme reacted with 2.5 mM nitrite at pH 6.4) was monitored by the rate of deoxyheme consumption. (F) First-order fits for β chains of HbA at pH 7.0 and for IHP-treated Hb at pH 6.4 for conditions in E showing that the deviation from first order requires an allosteric structural transition of the Hb tetramer (fits of natural log of deoxyheme concentration for the same reactions shown in E).
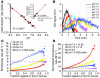
Figure 4
Maximal rates for nitrite reduction to NO occurs around the Hb P50. (A) The negative of the change in deoxyheme concentration (obtained by spectral deconvolution) over time during the reaction of nitrite (10 mM) with partially oxygenated (0–40%) Hb (50 μM total heme). (B) Instantaneous rate of deoxyheme consumption over the course of the experiment described in A. (C) Initial rate (intercept of the polynomial fitting in B at 0 seconds) of reaction for the conditions described above plotted as a function of initial oxygen saturation. (D) The instantaneous rate of deoxyheme consumption versus fraction ligated or oxidized ferric hemes (relative to total heme: deoxy-, met-, and iron-nitrosyl-Hb) during the course of the anaerobic reaction of nitrite (10 mM) with deoxyheme (50 μM).
Figure 5
Effect of pH on the nitrite reductase reaction. (A) The initial rate (found by the polynomial fitting of the rate of deoxyheme consumption over fraction ligated) of the reaction of nitrite (2.5 mM) with deoxyHb (45 μM heme) at different pH values in 0.01 M phosphate buffer. Inset shows the initial rate plotted against concentration of proton. (B) NO gas measured by chemiluminescence from the reactions shown in A. (C) The bimolecular rate of the reaction of nitrite with deoxyHb in phosphate buffer at pH 7.6, 7.0, and 6.5 and deoxymyoglobin in PBS buffer at pH 7.4 over heme ligand and oxidation states. (D) Correction factors 12.59 and 3.16 (given by 10pH – 6.5) were multiplied by the bimolecular rates at pH 7.6 and 7.0, respectively (shown in C) to eliminate the contribution of changing proton concentrations to the bimolecular rate.
Similar articles
- The oxidative denitrosylation mechanism and nitric oxide release from human fetal and adult hemoglobin, an experimentally based model simulation study.
Salhany JM. Salhany JM. Blood Cells Mol Dis. 2013 Jan;50(1):8-19. doi: 10.1016/j.bcmd.2012.08.006. Epub 2012 Sep 13. Blood Cells Mol Dis. 2013. PMID: 22981699 - The new chemical biology of nitrite reactions with hemoglobin: R-state catalysis, oxidative denitrosylation, and nitrite reductase/anhydrase.
Gladwin MT, Grubina R, Doyle MP. Gladwin MT, et al. Acc Chem Res. 2009 Jan 20;42(1):157-67. doi: 10.1021/ar800089j. Acc Chem Res. 2009. PMID: 18783254 Review. - The detection of the nitrite reductase and NO-generating properties of haemoglobin by mitochondrial inhibition.
Shiva S, Rassaf T, Patel RP, Gladwin MT. Shiva S, et al. Cardiovasc Res. 2011 Feb 15;89(3):566-73. doi: 10.1093/cvr/cvq327. Epub 2010 Oct 14. Cardiovasc Res. 2011. PMID: 20952414 Free PMC article. - Concerted nitric oxide formation and release from the simultaneous reactions of nitrite with deoxy- and oxyhemoglobin.
Grubina R, Huang Z, Shiva S, Joshi MS, Azarov I, Basu S, Ringwood LA, Jiang A, Hogg N, Kim-Shapiro DB, Gladwin MT. Grubina R, et al. J Biol Chem. 2007 Apr 27;282(17):12916-27. doi: 10.1074/jbc.M700546200. Epub 2007 Feb 23. J Biol Chem. 2007. PMID: 17322300 - The functional nitrite reductase activity of the heme-globins.
Gladwin MT, Kim-Shapiro DB. Gladwin MT, et al. Blood. 2008 Oct 1;112(7):2636-47. doi: 10.1182/blood-2008-01-115261. Epub 2008 Jul 2. Blood. 2008. PMID: 18596228 Free PMC article. Review.
Cited by
- Structural and functional properties of Antarctic fish cytoglobins-1: Cold-reactivity in multi-ligand reactions.
Giordano D, Pesce A, Vermeylen S, Abbruzzetti S, Nardini M, Marchesani F, Berghmans H, Seira C, Bruno S, Javier Luque F, di Prisco G, Ascenzi P, Dewilde S, Bolognesi M, Viappiani C, Verde C. Giordano D, et al. Comput Struct Biotechnol J. 2020 Aug 12;18:2132-2144. doi: 10.1016/j.csbj.2020.08.007. eCollection 2020. Comput Struct Biotechnol J. 2020. PMID: 32913582 Free PMC article. - Nitrite regulates hypoxic vasodilation via myoglobin-dependent nitric oxide generation.
Totzeck M, Hendgen-Cotta UB, Luedike P, Berenbrink M, Klare JP, Steinhoff HJ, Semmler D, Shiva S, Williams D, Kipar A, Gladwin MT, Schrader J, Kelm M, Cossins AR, Rassaf T. Totzeck M, et al. Circulation. 2012 Jul 17;126(3):325-34. doi: 10.1161/CIRCULATIONAHA.111.087155. Epub 2012 Jun 9. Circulation. 2012. PMID: 22685116 Free PMC article. - Tandem P-selectin glycoprotein ligand immunoglobulin prevents lung vaso-occlusion in sickle cell disease mice.
Vats R, Tutuncuoglu E, Pradhan-Sundd T, Tejero J, Shaw GD, Sundd P. Vats R, et al. Exp Hematol. 2020 Apr;84:1-6.e1. doi: 10.1016/j.exphem.2020.03.002. Epub 2020 Mar 31. Exp Hematol. 2020. PMID: 32243995 Free PMC article. - Red Blood Cell Dysfunction in Critical Illness.
Rogers S, Doctor A. Rogers S, et al. Crit Care Clin. 2020 Apr;36(2):267-292. doi: 10.1016/j.ccc.2019.12.008. Epub 2020 Feb 11. Crit Care Clin. 2020. PMID: 32172813 Free PMC article. Review. - Hemoglobin inhibits albumin uptake by proximal tubule cells: implications for sickle cell disease.
Eshbach ML, Kaur A, Rbaibi Y, Tejero J, Weisz OA. Eshbach ML, et al. Am J Physiol Cell Physiol. 2017 Jun 1;312(6):C733-C740. doi: 10.1152/ajpcell.00021.2017. Epub 2017 Mar 29. Am J Physiol Cell Physiol. 2017. PMID: 28356267 Free PMC article.
References
- Tune JD, Richmond KN, Gorman MW, Feigl EO. K(ATP)(+) channels, nitric oxide, and adenosine are not required for local metabolic coronary vasodilation. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H868–H875. - PubMed
- Tune JD, Gorman MW, Feigl EO. Matching coronary blood flow to myocardial oxygen consumption. J. Appl. Physiol. 2004;97:404–415. - PubMed
- Ellsworth ML. The red blood cell as an oxygen sensor: what is the evidence [review]? Acta Physiol. Scand. 2000;168:551–559. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL058091/HL/NHLBI NIH HHS/United States
- R37 HL058091/HL/NHLBI NIH HHS/United States
- HL58091/HL/NHLBI NIH HHS/United States
- R01 GM055792/GM/NIGMS NIH HHS/United States
- R29 GM055792/GM/NIGMS NIH HHS/United States
- GM55792/GM/NIGMS NIH HHS/United States
- R29 HL058091/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials