A {gamma}-secretase-independent mechanism of signal transduction by the amyloid precursor protein - PubMed (original) (raw)
A {gamma}-secretase-independent mechanism of signal transduction by the amyloid precursor protein
Matthew R Hass et al. J Biol Chem. 2005.
Abstract
It has been proposed that gamma-secretase-mediated release of the amyloid precursor protein (APP) intracellular domain (AICD) results in nuclear translocation and signaling through a complex with the adaptor protein Fe65 and the histone acetyltransferase Tip60. Here, we show that APP and Fe65 activate transcription through a Gal4-Tip60 reporter in presenilin-1/2-deficient cells lacking generation of AICD. APP and Fe65 also activated transcription in the presence of gamma-secretase inhibitors that prevent amyloid beta-peptide production in human embryonic kidney 293 and SH-SY5Y cells. In contrast to the transcriptionally active Notch intracellular domain, expression of AICD did not activate transcription. An alternative mechanism for APP signal transduction is suggested by the identification of essential cyclin-dependent kinase (CDK) phosphorylation sites in Tip60. Mutation of these Tip60 phosphorylation sites or treatment with the CDK inhibitor roscovitine blocked the ability of APP to signal through Tip60. Moreover, APP stabilized Tip60 through CDK-dependent phosphorylation. Subcellular fractionation and confocal immunofluorescence showed that APP recruited Tip60 to membrane compartments. Thus, APP may signal to the nucleus by a gamma-secretase-independent mechanism that involves membrane sequestration and phosphorylation of Tip60.
Figures
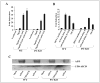
FIGURE 1. APP signaling does not require γ-secretase cleavage, in contrast to Notch signaling
A, wild-type (WT) ES or PS-KO cells were cotransfected in triplicate with the pFR-luciferase, pRL-TK, Gal4-Tip60, Fe65, APP, and PS1 plasmids as indicated. Approximately 24 h after transfection, the cells were collected, and dual-luciferase reporter assays were performed. B, wild-type ES or PS-KO cells were cotransfected in triplicate with HES1-luciferase, pRL-TK, mNotchΔE, PS1, and NICD as indicated, and luciferase assays were performed at 24 h post-transfection. C, wild-type ES or PS-KO cells were transfected with the pcDNA (Mock), APPwt, C50/AICD, and APPsw plasmids. The cells were harvested 24 h after transfection, separated on a 10 –20% Tris/Tricine gel, and Western-blotted with antibody C8 (directed against the APP C-terminal domain).
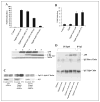
FIGURE 2. γ-Secretase inhibitors do not affect APP signaling, and AICD does not have transcriptional activity
A (upper panel) and B, HEK-293 and SH-SY5Y cells, respectively, were cotransfected in triplicate with pFR-luciferase, pRL-TK, Gal4-Tip60, Fe65, APP, and C50-Myc as indicated. After 3 h, the γ-secretase inhibitor WPE-II-72 (I-72, I72; 500 n
m
) was added. Luciferase assays were performed on cell lysates at 24 h after transfection. A (lower panel), cell lysates were Western-blotted for APP with antibody C8 and for actin as a loading control. C, HEK-293 cells stably expressing APPsw or HEK-293 and SH-SY5Y cells transiently transfected with Tip60, Fe65, and APPwt were incubated in the presence or absence (Control) of the γ-secretase inhibitor WPE-II-72 (500 n
m
). Aβ was immunoprecipitated with antibody 159 and Western-blotted with antibody 6E10. D, HEK-293 cells were transfected with wild-type Tip60 (Tip60wt) or the Tip60(S86A/S90A) mutant (Tip60s86/90a) together with Fe65 or APP as indicated. Tip60 was immunoprecipitated (IP) with anti-HA antibody, and the co-immunoprecipitated APP was detected by Western blotting with antibody C8.
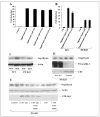
FIGURE 3. APP signaling is independent of caspase cleavage and does not regulate neprilysin levels
A and B, HEK-293 cells and wild-type (WT) ES and PS-KO cells, respectively, were cotransfected with pFR-luciferase (Luc), pRL-TK, Gal4-Tip60, Fe65, and APP as indicated. Cells were treated 4 h post-transfection with the γ-secretase inhibitor WPE-II-72 (I72; 500 n
m
) and with the caspase inhibitor benzyloxycarbonyl-VAD-fluoromethyl ketone (ZVAD; 20 μ
m
). Luciferase assays were performed 24 h after transfection. C, whole cell lysates from two independent wild-type ES (PBD6 and PBD8) and two PS-KO (BD8 and BD35) cell lines were probed from neprilysin and then reprobed for actin. D, BD8 PS-KO cells were transfected with PS1, and lysates were collected 24 h later and Western-blotted in duplicate for neprilysin, presenilin, and APP C-terminal fragments. E, BD8 PS-KO cells were transfected with either C50 or C50-Myc alone or with Tip60 and Fe65. After 24 h, the cells were harvested and Western-blotted for neprilysin, actin, and C50-Myc expression. (C50 expression was confirmed on a darker exposure (data not shown).)
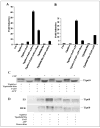
FIGURE 4. CDK phosphorylation of Tip60 is required for APP-dependent transactivation and stabilization of Tip60
A and B, luciferase assays were performed on HEK-293 and wild-type ES cells, respectively, transfected with Gal4-Tip60 (Tip60wt) or Gal4-Tip60(S86A/S90A) (Tip60s86/ 90a) together with Fe65 and APP. Cells were treated with the CDK inhibitor roscovitine (Rosc.; 20 μ
m
) as indicated for 24 h. Luc, luciferase. C, shown is the phosphorylation of Tip60. HEK-293 cells were cotransfected with wild-type Tip60 (Tip60wt) or Tip60(S86A/S90A) (Tip60s86/90a) alone or with Fe65 and APP. Cells were harvested at 24 h post-transfection, and equivalent amounts of lysate protein were incubated in the absence or presence of calf intestinal alkaline phosphatase (CIP) and then Western-blotted with anti-Tip60 antibody N1. D, APP-dependent stabilization of Tip60 requires CDK phosphorylation. Wild-type ES and HEK-293 cells were cotransfected with wild-type Tip60 or Tip60(S86A/S90A), Fe65, and APP and treated with roscovitine (20 μ
m
).
FIGURE 5. APP recruits Tip60 to a membrane compartment in vitro and in vivo
A, HEK-293 cells were transfected with wild-type Tip60 (Tip60wt) alone or with Fe65 and APP; and 24 h after transfection, the cells were subjected to sub-cellular fractionation into nuclear, cytoplasmic, and membrane fractions. The fractions were Western-blotted for Tip60, which showed membrane recruitment by coexpression of Fe65 and APP. Western blotting for the nuclear protein lamin B2 confirmed the lack of nuclear contamination of the membrane fraction. B, similar results were observed with endogenous Tip60 in HEK-293 cells stably expressing APP. C, cortices and hippocampi were isolated from APP transgenic and littermate control (wild-type (Wt)) mice that were either 3 months (young) or 2 years (old) of age. Cortical and hippocampal samples were then fractionated, and Western blotting was performed for Tip60.
FIGURE 6. Colocalization of APP and Tip60
SH-SY5Y cells were cotransfected with Tip60 alone (A–C) or with Fe65 and APP (D–F) and then fixed and permeabilized at 24 h post-transfection. Tip60 was visualized with an antibody against the HA tag and a Cy3-conjugated secondary antibody (red), and APP was visualized with antibody C8 and a Cy2-conjugated secondary antibody (green). The nucleus was labeled with Hoechst dye.
FIGURE 7. Shuttling of Tip60 between the nucleus and cytoplasm and APP signaling
A, identification of two potential nuclear localization sequences (NLS) within Tip60 are conserved in the mouse, rat, and human orthologs. Additionally, a conserved potential nuclear export sequence is indicated. B, HEK-293 cells were cotransfected with pFR-luciferase, pRL-TK, Gal4-Tip60, Fe65, and APP. Cells were treated with the nuclear export inhibitor leptomycin B (20 n
m
), and luciferase assays were performed 24 after transfection.
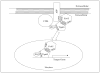
FIGURE 8. Model of APP-mediated activation of Tip60
APP recruits Tip60 to the membrane through the adaptor protein Fe65. This facilitates the phosphorylation and stabilization of Tip60 by CDKs, resulting in Tip60 activation. This is followed by dissociation of the complex and nuclear translocation of Tip60 and Fe65, leading to the transcriptional activation of Tip60 target genes. PTB, phosphotyrosine-binding domain.
Similar articles
- Dissection of amyloid-beta precursor protein-dependent transcriptional transactivation.
Cao X, Südhof TC. Cao X, et al. J Biol Chem. 2004 Jun 4;279(23):24601-11. doi: 10.1074/jbc.M402248200. Epub 2004 Mar 24. J Biol Chem. 2004. PMID: 15044485 - Notch1 intracellular domain suppresses APP intracellular domain-Tip60-Fe65 complex mediated signaling through physical interaction.
Kim SY, Kim MY, Mo JS, Park HS. Kim SY, et al. Biochim Biophys Acta. 2007 Jun;1773(6):736-46. doi: 10.1016/j.bbamcr.2007.02.001. Epub 2007 Feb 14. Biochim Biophys Acta. 2007. PMID: 17368826 - Visualization and quantification of APP intracellular domain-mediated nuclear signaling by bimolecular fluorescence complementation.
Riese F, Grinschgl S, Gersbacher MT, Russi N, Hock C, Nitsch RM, Konietzko U. Riese F, et al. PLoS One. 2013 Sep 25;8(9):e76094. doi: 10.1371/journal.pone.0076094. eCollection 2013. PLoS One. 2013. PMID: 24086696 Free PMC article. - A putative role of the Amyloid Precursor Protein Intracellular Domain (AICD) in transcription.
Słomnicki LP, Leśniak W. Słomnicki LP, et al. Acta Neurobiol Exp (Wars). 2008;68(2):219-28. doi: 10.55782/ane-2008-1691. Acta Neurobiol Exp (Wars). 2008. PMID: 18511958 Review. - Role of presenilin in gamma-secretase cleavage of amyloid precursor protein.
Xia W. Xia W. Exp Gerontol. 2000 Jul;35(4):453-60. doi: 10.1016/s0531-5565(00)00111-x. Exp Gerontol. 2000. PMID: 10959033 Review.
Cited by
- Neprilysin and Aβ Clearance: Impact of the APP Intracellular Domain in NEP Regulation and Implications in Alzheimer's Disease.
Grimm MO, Mett J, Stahlmann CP, Haupenthal VJ, Zimmer VC, Hartmann T. Grimm MO, et al. Front Aging Neurosci. 2013 Dec 23;5:98. doi: 10.3389/fnagi.2013.00098. Front Aging Neurosci. 2013. PMID: 24391587 Free PMC article. Review. - Loss of gamma-secretase function impairs endocytosis of lipoprotein particles and membrane cholesterol homeostasis.
Tamboli IY, Prager K, Thal DR, Thelen KM, Dewachter I, Pietrzik CU, St George-Hyslop P, Sisodia SS, De Strooper B, Heneka MT, Filippov MA, Müller U, van Leuven F, Lütjohann D, Walter J. Tamboli IY, et al. J Neurosci. 2008 Nov 12;28(46):12097-106. doi: 10.1523/JNEUROSCI.2635-08.2008. J Neurosci. 2008. PMID: 19005074 Free PMC article. - FE65 binds Teashirt, inhibiting expression of the primate-specific caspase-4.
Kajiwara Y, Akram A, Katsel P, Haroutunian V, Schmeidler J, Beecham G, Haines JL, Pericak-Vance MA, Buxbaum JD. Kajiwara Y, et al. PLoS One. 2009;4(4):e5071. doi: 10.1371/journal.pone.0005071. Epub 2009 Apr 3. PLoS One. 2009. PMID: 19343227 Free PMC article. - Neurodegeneration in Alzheimer disease: role of amyloid precursor protein and presenilin 1 intracellular signaling.
Nizzari M, Thellung S, Corsaro A, Villa V, Pagano A, Porcile C, Russo C, Florio T. Nizzari M, et al. J Toxicol. 2012;2012:187297. doi: 10.1155/2012/187297. Epub 2012 Feb 8. J Toxicol. 2012. PMID: 22496686 Free PMC article. - The amyloid precursor protein intracellular domain-fe65 multiprotein complexes: a challenge to the amyloid hypothesis for Alzheimer's disease?
Bórquez DA, González-Billault C. Bórquez DA, et al. Int J Alzheimers Dis. 2012;2012:353145. doi: 10.1155/2012/353145. Epub 2012 Feb 9. Int J Alzheimers Dis. 2012. PMID: 22506131 Free PMC article.
References
- Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. Nature. 1987;325:733–736. - PubMed
- Selkoe D, Kopan R. Annu Rev Neurosci. 2003;26:565–597. - PubMed
- De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. Nature. 1999;398:518–522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG0022/AG/NIA NIH HHS/United States
- T32 AG000222/AG/NIA NIH HHS/United States
- R01 NS030352/NS/NINDS NIH HHS/United States
- R01 AG017974/AG/NIA NIH HHS/United States
- AG17974/AG/NIA NIH HHS/United States
- NS030352/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous