p63 deficiency activates a program of cellular senescence and leads to accelerated aging - PubMed (original) (raw)
. 2005 Sep 1;19(17):1986-99.
doi: 10.1101/gad.342305. Epub 2005 Aug 17.
Affiliations
- PMID: 16107615
- PMCID: PMC1199570
- DOI: 10.1101/gad.342305
p63 deficiency activates a program of cellular senescence and leads to accelerated aging
William M Keyes et al. Genes Dev. 2005.
Abstract
The p53 tumor suppressor plays a key role in organismal aging. A cellular mechanism postulated to drive the aging process is cellular senescence, mediated in part by p53. Although senescent cells accumulate in elderly individuals, most studies have relied on correlating in vitro senescence assays with in vivo phenotypes of aging. Here, using two different mouse models in which the p53-related protein p63 is compromised, we demonstrate that cellular senescence and organismal aging are intimately linked and that these processes are mediated by p63 loss. We found that p63(+/-) mice have a shortened life span and display features of accelerated aging. Both germline and somatically induced p63 deficiency activates widespread cellular senescence with enhanced expression of senescent markers SA-beta-gal, PML, and p16(INK4a). Using an inducible tissue-specific p63 conditional model, we further show that p63 deficiency induces cellular senescence and causes accelerated aging phenotypes in the adult. Our results thus suggest a causative link between cellular senescence and aging in vivo, and demonstrate that p63 deficiency accelerates this process.
Figures
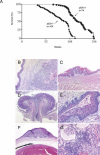
Figure 1.
Life span is decreased in p63+/- mice. (A) Over the course of a >2.2-yr study, the median life span for wild-type mice (n = 74) was 121 wk, whereas that of p63+/- mice (n = 104) was 95 wk, a difference of 21.5% (P < 0.0001, log-rank test). p63+/- mice frequently developed hyperplastic lesions in a variety of epithelia. (_B_-G) Histological analysis of tissues of p63+/- mice. (B) Dorsal back skin. (C) Dorsal surface of the tongue. (D) Rectum. (E) Vagina. (F) Cornea of the eye. (G) Glomerulus of the kidney.
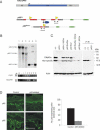
Figure 2.
Inducible, tissue-specific Cre-mediated p63 disruption in vivo. (A) Schematic diagram of the K5CrePR1 and p63flox alleles used to generate the p63 conditional system. The K5CrePR1 transgene uses the K5 promoter to drive expression of the Cre-progesterone receptor fusion protein, which provides expression of an inducible form of Cre specifically within stratified epithelia such as the skin. The p63flox allele contains a pair of loxP sites (yellow triangles) encompassing the majority of the DBD (red). Excision of DBD-encoding exons by Cre disrupts p63, alters the reading frame of any potential p63 transcript, and inactivates all p63 isoforms. p63flox and p63Brdm3 (disrupted) alleles were identified by amplifying the unique junctions using the PCR primers shown (arrows). (B, top) Southern blot hybridization of DNA samples from E17.5 embryos showing endogenous (p63), null (p63Brdm2), and floxed (p63flox) p63 alleles. (Bottom) PCR was used to amplify β-actin as a positive control and the K5CrePR1 transgene from the same templates analyzed above. p63flox/Brdm2; K5CrePR1 (lane 5) and p63flox/flox; K5CrePR1 (lane 6) have the correct genotype to facilitate Cre-mediated p63 disruption in response to mifepristone treatment. (C) Western blot analyses of lysates prepared from dorsal back skin biopsies obtained from E17.5 wild-type and _p63_-/- embryos, as well as from embryos that had been treated with mifepristone at E10.5. DNp63α, the predominant p63 isoform in mature epidermis, is efficiently ablated in utero. (D) p63 immunofluorescence and quantitation of p63-expressing cells from post-natal day 4 (P4) dorsal back skin from control (p63Brdm2/flox) and p63-ablated (p63flox/Brdm2; K5CrePR1) mice after treatment with inducer at P2. Quantitation of proliferating cells was evaluated by counting the number of fluorescently labeled cells per total number of cells in both the interfollicular and follicular epidermis. Bar, 100 μM.
Figure 3.
Cre-mediated p63 disruption induced by mifepristone treatment efficiently ablates p63 in utero. (A) Whole-mount microscopy of control, _p63_-/-, and p63-ablated embryos at E17.5 demonstrates that p63-ablated mice have a phenotype similar to that of _p63_-/- embryos. Note that the p63-ablated embryo shown was generated by administration of mifepristone at E8.5, and the embryo was allowed to develop until harvest at E17.5. (B) Skin biopsies from control and p63-ablated embryos similar to those shown in A were examined by histological and immunofluorescent analyses. Epidermal morphogenesis was halted in response to Cre-mediated p63 ablation induced by mifepristone treatment at E8.5, as seen by the lack of stratification by histological analysis (H&E) and by the absence of expression of proliferating (K14), early-stage (K1/K10), and late-stage (fillaggrin) epidermal markers by immunofluorescence in p63-ablated skin. Bar, 10 μM.
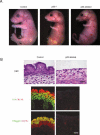
Figure 4.
The senescence marker SA-β-gal is induced in response to both germline and somatically induced p63 deficiency in vivo, and in primary keratinocytes in culture. (A) A whole-mount assay for endogenous SA-β-gal activity was performed on E17.5 p63+/+, _p63_-/-, and p63-ablated embryos; skin sections from SA-β-gal assayed embryos were subjected to histological analysis; the intense blue color observed in _p63_-/- embryos is indicative of senescent cells. Bar, 100 μM. (B) Dorsal back skin biopsies from E17.5 control, _p63_-/-, and p63-ablated embryos (treated with mifepristone at E8.5) were stained with Dapi to show histology (bottom panels) and assayed for endogenous SA-β-gal activity (top panels). Positive (blue) cells were essentially undetectable in control skin, whereas _p63_-/- and p63-ablated skin had a dramatic increase in cellular senescence. Bar, 100 μM. (C) Primary keratinocyte cultures from p63flox/flox mice were uninfected, or infected with GFP-expressing (GFP) or Cre-expressing (Cre) retrovirus. DNA prepared from these cultures was subjected to PCR to identify the disrupted p63Brdm3 allele. β-Actin was used as a positive control. Cultures were subjected to the SA-β-gal assay, and senescent cells were quantitated. Bar, 100 μm.
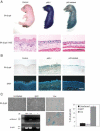
Figure 5.
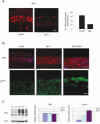
Key mediators of cellular senescence are induced in response to p63 deficiency in utero. (A) In vivo proliferation was assessed in E17.5 embryos using the BrdU incorporation assay. After an in utero pulse with BrdU, embryos were harvested and dorsal back skin biopsies from p63+/+ (Control) and _p63_-/- embryos were subjected to immunofluorescent analysis to detect cells that incorporated BrdU. (Right) Proliferating cells were quantitated, showing that p63 deficiency causes decreased proliferation in vivo (±SEM; P < 0.001). Bar, 10 μm. (B) Immunofluorescence for PML and p16INK4a in p63+/+, _p63_-/-, and p63-ablated embryonic skin at E17.5. Bars represent 10 μm for p16INK4a and 50 μm for PML. (C) Western blot analysis for PML in representative p63+/+ (wt), _p63_-/- (-/-), and p63-ablated (ab) skin lysates. Real-time PCR analysis was used to determine PML and p16INK4a expression at the transcript level.
Figure 6.
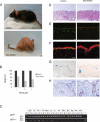
Aging features and senescence-associated markers are induced in response to p63 deficiency in adult mice. (A) Cre-mediated ablation of p63 in vivo in control (top panel) and ablated (bottom panel) adult mice at 8 mo of age. Note the pronounced lordokyphosis and alopecia caused by p63 deficiency. (B) Weight of age- and sex-matched sibling pairs of control and p63-ablated adult mice at 7, 20, and 22 mo of age, expressed as a percentage of control. (C) The tissue specificity of Cre-mediated p63 disruption in adult control (c), ablated (a) mice was evaluated by PCR amplification of the p63flox and p63Brdm3 alleles from genomic DNA obtained from different tissues. (Sk) Skin; (To) tongue; (Fs) forestomach; (Th) thymus; (Mu) muscle; (Lu) lung; (Li) liver; (Ki) kidney; (Re) reproductive tract (female); (Bf) brown fat; (Sp) spleen; (He) heart; (Br) brain; (m) BstEII-digested λ DNA; (-) negative water control; (+) genomic DNA from a p63Brdm3/+ mouse. (_D_-G) Dorsal back skin sections of control and p63-ablated 8-mo-old mice, showing the pronounced aging-associated features in the skin of p63-ablated mice. (D) Hematoxylin and eosin staining. (E) K14 (green) and K1/K10 (red). (F) p63 (green) and K1/K10 (red). (G) SA-β-gal activity. (H) p16INK4a expression. Bars: D,E,G,H, 100 μm; F, 10 μm.
Figure 7.
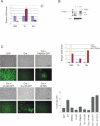
Senescence in p63-ablated keratinocytes is prevented by knock-down of p53 and PML, but not by p16INK4a knock-down or ectopic DNp63α overexpression. (A) Real-time PCR analysis for the DBD, TA, and N-terminal truncated (DN) transcripts in response to Cre-mediated ablation in primary keratinocytes. (B) Western blot analysis for p63 in GFP-infected (V), Cre-infected (C), or DNp63α-infected (DN) keratinocytes, showing that the DNp63α isoform (arrow) and other putative p63 isoforms (dashed arrows) are efficiently ablated in Cre-infected cultures. Asterisk denotes nonspecific band. (C, top right) Real-time PCR analysis for transcripts encoding different p63 isoform subtypes in keratinocytes infected with GFP, DNp63α, or Cre + DNp63α. (Left) Phenotypic and fluorescent analyses of primary keratinocyte cultures infected with GFP- or Cre-expressing vectors, or with Cre in combination with DNp63α, or with Cre in combination with short hairpins specific for p53, p16INK4a, or PML. The proliferation defect was prevented in cultures treated with sh p53 and sh PML, but not sh p16 or DNp63α. (Bottom right) Cultures were assayed for SA-β-gal activity following infection, and the percentage of positive cells was quantitated. Bars, 100 μm.
Similar articles
- TAp63 induces senescence and suppresses tumorigenesis in vivo.
Guo X, Keyes WM, Papazoglu C, Zuber J, Li W, Lowe SW, Vogel H, Mills AA. Guo X, et al. Nat Cell Biol. 2009 Dec;11(12):1451-7. doi: 10.1038/ncb1988. Epub 2009 Nov 8. Nat Cell Biol. 2009. PMID: 19898465 Free PMC article. - p63: a new link between senescence and aging.
Keyes WM, Mills AA. Keyes WM, et al. Cell Cycle. 2006 Feb;5(3):260-5. doi: 10.4161/cc.5.3.2415. Epub 2006 Feb 1. Cell Cycle. 2006. PMID: 16434880 Review. - SKP-ing TAp63: stem cell depletion, senescence, and premature aging.
Beaudry VG, Attardi LD. Beaudry VG, et al. Cell Stem Cell. 2009 Jul 2;5(1):1-2. doi: 10.1016/j.stem.2009.06.015. Cell Stem Cell. 2009. PMID: 19570504 - p63, cellular senescence and tumor development.
Guo X, Mills AA. Guo X, et al. Cell Cycle. 2007 Feb 1;6(3):305-11. doi: 10.4161/cc.6.3.3794. Epub 2007 Feb 3. Cell Cycle. 2007. PMID: 17224650 Review. - Differential expression of p63 isoforms in female reproductive organs.
Kurita T, Cunha GR, Robboy SJ, Mills AA, Medina RT. Kurita T, et al. Mech Dev. 2005 Sep;122(9):1043-55. doi: 10.1016/j.mod.2005.04.008. Mech Dev. 2005. PMID: 15922574
Cited by
- Tracing the protectors path from the germ line to the genome.
Coutandin D, Ou HD, Löhr F, Dötsch V. Coutandin D, et al. Proc Natl Acad Sci U S A. 2010 Aug 31;107(35):15318-25. doi: 10.1073/pnas.1001069107. Epub 2010 Aug 9. Proc Natl Acad Sci U S A. 2010. PMID: 20696896 Free PMC article. - TAp63 induces senescence and suppresses tumorigenesis in vivo.
Guo X, Keyes WM, Papazoglu C, Zuber J, Li W, Lowe SW, Vogel H, Mills AA. Guo X, et al. Nat Cell Biol. 2009 Dec;11(12):1451-7. doi: 10.1038/ncb1988. Epub 2009 Nov 8. Nat Cell Biol. 2009. PMID: 19898465 Free PMC article. - ΔNp63α promotes cellular quiescence via induction and activation of Notch3.
Kent S, Hutchinson J, Balboni A, Decastro A, Cherukuri P, Direnzo J. Kent S, et al. Cell Cycle. 2011 Sep 15;10(18):3111-8. doi: 10.4161/cc.10.18.17300. Epub 2011 Sep 15. Cell Cycle. 2011. PMID: 21912215 Free PMC article. - Age and Sex: Impact on adipose tissue metabolism and inflammation.
Varghese M, Song J, Singer K. Varghese M, et al. Mech Ageing Dev. 2021 Oct;199:111563. doi: 10.1016/j.mad.2021.111563. Epub 2021 Aug 30. Mech Ageing Dev. 2021. PMID: 34474078 Free PMC article. Review. - Epithelial cell senescence: an adaptive response to pre-carcinogenic stresses?
Abbadie C, Pluquet O, Pourtier A. Abbadie C, et al. Cell Mol Life Sci. 2017 Dec;74(24):4471-4509. doi: 10.1007/s00018-017-2587-9. Epub 2017 Jul 13. Cell Mol Life Sci. 2017. PMID: 28707011 Free PMC article. Review.
References
- Bernardi R., Scaglioni, P.P., Bergmann, S., Horn, H.F., Vousden, K.H., and Pandolfi, P.P. 2004. PML regulates p53 stability by sequestering Mdm2 to the nucleolus. Nat. Cell Biol. 6: 665-672. - PubMed
- Campisi J. 2001. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 11: S27-S31. - PubMed
- ____. 2005. Senescent cells, tumor suppression, and organismal aging: Good citizens, bad neighbors. Cell 120: 513-522. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous