Francisella tularensis enters macrophages via a novel process involving pseudopod loops - PubMed (original) (raw)
Francisella tularensis enters macrophages via a novel process involving pseudopod loops
Daniel L Clemens et al. Infect Immun. 2005 Sep.
Abstract
Intracellular bacterial pathogens employ a variety of strategies to invade their eukaryotic host cells. From an ultrastructural standpoint, the processes that bacteria employ to invade their host cells include conventional phagocytosis, coiling phagocytosis, and ruffling/triggered macropinocytosis. In this paper, we describe a novel process by which Francisella tularensis, the agent of tularemia, enters host macrophages. F. tularensis is a remarkably infectious facultative intracellular bacterial parasite--as few as 10 bacteria can cause life-threatening disease in humans. However, the ultrastructure of its uptake and the receptor mechanisms that mediate its uptake have not been reported previously. We have used fluorescence microscopy and electron microscopy to examine the adherence and uptake of a virulent recent clinical isolate of F. tularensis, subspecies tularensis, and the live vaccine strain (LVS), subspecies holarctica, by human macrophages. We show here that both strains of F. tularensis enter human macrophages by a novel process of engulfment within asymmetric, spacious pseudopod loops, a process that differs ultrastructurally from all previously described uptake mechanisms. We demonstrate also that adherence and uptake of F. tularensis by macrophages is strongly dependent upon complement receptors and upon serum with intact complement factor C3 and that uptake requires actin microfilaments. These findings have significant implications for understanding the intracellular biology and virulence of this extremely infectious pathogen.
Figures
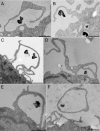
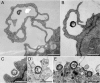
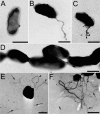
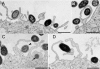
FIG. 1.
Human MDM engulf F. tularensis within spacious pseudopod loops. Live F. tularensis RCI were preoposonized with 10% fresh human serum, pelleted onto human peripheral blood MDM (A, B, and D to F) or differentiated THP-1 cells (C), fixed, and processed for TEM after 5 min of incubation at 37°C. Similar results were also obtained with F. tularensis LVS. Size bars indicate 1 μm in all panels.
FIG. 2.
TEM demonstrates that within 5 min of uptake, the majority (90%) of F. tularensis RCI (A to C) and LVS (D) that are located within the main cell body of the MDM reside within tightly fitting vacuoles. In approximately 1% of F. tularensis RCI (B and C) and LVS (D) vacuoles, pili or protrusions of the bacterial cell wall appear to be in direct contact with the phagosomal walls. Size bars indicate 0.5 μm in all panels.
FIG. 3.
Interaction of formalin-killed and periodate-treated F. tularensis with human MDM. (A and B) The majority of formalin-killed F. tularensis bacteria are internalized by human MDM within exuberant pseudopod loops that resemble those of live bacteria. (C) A low percentage (4%) of the bacteria are internalized by conventional phagocytosis. (D and E) The majority (76%) of periodate-treated F. tularensis are internalized by conventional phagocytosis. Size bars indicate 1 μm in all panels.
FIG. 4.
TEM of negatively stained preparations of F. tularensis RCI (A to D) and a clinical isolate of N. gonorrhea (E and F) after overnight growth on agar plates. (A and D) The majority of F. tularensis RCI have no apparent pili, and the dimensions of the bacteria observed by negative staining of whole bacteria are similar to the dimensions of the bacteria observed in thin sections. (B and C) A low percentage of F. tularensis RCI possess pili, but the dimensions and distribution of these pili do not account for the spacious pseudopod loops observed during uptake by macrophages. (D) F. tularensis bacteria are frequently observed to be closely juxtaposed, arguing against the presence of large capsules that might be invisible in the negative staining. (E and F) In contrast to plate-grown F. tularensis RCI, the majority of organisms of a recent clinical isolate of N. gonorrhea exhibit fine type IV pili (arrows) and some also have thicker sex pili similar to those of _F. tulare_nsis (E) (arrowheads). Size bars indicate 0.5 μm in all panels.
FIG. 5.
S. enterica serovar Typhimurium (A to C) and S. flexneri (D) are internalized by human MDM by an ultrastructural process that differs from that of F. tularensis. S. enterica serovar Typhimurium bacteria (A and C) (arrowhead) are internalized within symmetric pairs of pseudopodia that are shorter than the pseudopod loops that engulf F. tularensis. Pseudopod loops (B) and vacuoles located at the periphery of the macrophage (C) (arrow) are less spacious than those observed for F. tularensis. (D) Uptake of S. flexneri within a symmetric pair of pseudopodia. Size bars indicate 1 μm in all panels.
FIG. 6.
Cytochalasin B inhibits the internalization of F. tularensis by human THP-1 cells. THP-1 cells were pretreated for 15 min with the indicated concentration of cytochalasin B prior to incubation for 60 min with F. tularensis RCI at a MOI of 35:1 (bacteria:macrophage) in the same concentration of cytochalasin B and 10% serum in RPMI 1640. Monolayers were washed, fixed, stained by immunofluorescence for extracellular bacteria, permeabilized, and stained for intracellular bacteria with a different fluorescent antibody. Bacteria that were stained by fluorescent antibody only after permeabilization were counted as internalized (▪); bacteria stained by fluorescent antibody prior to permeabilization were counted as external (□). Data shown are mean values (± standard errors [SE]) of triplicate counts of more than 300 macrophages each. We obtained similar results with MDM and with F. tularensis LVS and with formalin-killed F. tularensis.
FIG.7.
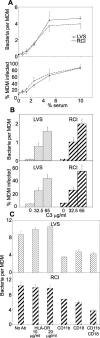
Adherence/uptake of F. tularensis by human macrophages requires serum, complement factor C3, and complement receptors. (A) Monolayers of human MDM on glass coverslips were incubated with F. tularensis live vaccine strain (LVS) or a virulent recent clinical isolate (RCI) in culture medium containing 10% HI-FBS and 0 to 10% autologous serum at an MOI of 35:1 for 1 h at 37°C. Monolayers were washed to remove nonadherent bacteria, fixed, and stained by immunofluorescence for F. tularensis. The number of adherent or internalized bacteria per MDM (top panel) and the percentage of MDM with adherent or internalized bacteria (bottom panel) were determined by immunofluorescence microscopy. Values represent the means (± SE) of triplicate determinations of at least 100 MDM examined for each data point. (B) Monolayers of human MDM on glass coverslips were incubated with F. tularensis live vaccine strain (LVS) or a virulent recent clinical isolate (RCI) at an MOI of 35:1 in culture medium containing 10% C3-depleted human AB serum supplemented with 0, 32.5, or 65 μg/ml of purified C3, and the numbers of bacteria per macrophage were determined as described above. (C) Monolayers of human MDM were treated with or without mouse anti-human complement receptor antibodies (anti-CD11b and/or anti-CD18) or an isotypic control antibody (anti-HLA-DR) for 30 min before incubation with F. tularensis LVS or RCI and with the same monoclonal antibody (all antibodies at 10 μg/ml) in the culture medium with 10% AB serum for 60 min at 37°C. Monolayers were washed, fixed, stained, and viewed by immunofluorescence microscopy to determine the number of bacteria per macrophage as described above. Values shown represent means (± SE) of triplicate determinations of at least 100 macrophages each.
Similar articles
- Francisella tularensis Confronts the Complement System.
Brock SR, Parmely MJ. Brock SR, et al. Front Cell Infect Microbiol. 2017 Dec 19;7:523. doi: 10.3389/fcimb.2017.00523. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29312899 Free PMC article. - Virulent and avirulent strains of Francisella tularensis prevent acidification and maturation of their phagosomes and escape into the cytoplasm in human macrophages.
Clemens DL, Lee BY, Horwitz MA. Clemens DL, et al. Infect Immun. 2004 Jun;72(6):3204-17. doi: 10.1128/IAI.72.6.3204-3217.2004. Infect Immun. 2004. PMID: 15155622 Free PMC article. - Francisella tularensis travels a novel, twisted road within macrophages.
Santic M, Molmeret M, Klose KE, Abu Kwaik Y. Santic M, et al. Trends Microbiol. 2006 Jan;14(1):37-44. doi: 10.1016/j.tim.2005.11.008. Epub 2005 Dec 13. Trends Microbiol. 2006. PMID: 16356719 Review. - Uptake and intracellular fate of Francisella tularensis in human macrophages.
Clemens DL, Horwitz MA. Clemens DL, et al. Ann N Y Acad Sci. 2007 Jun;1105:160-86. doi: 10.1196/annals.1409.001. Epub 2007 Apr 13. Ann N Y Acad Sci. 2007. PMID: 17435118 Review.
Cited by
- An in vitro model system used to study adherence and invasion of Francisella tularensis live vaccine strain in nonphagocytic cells.
Lindemann SR, McLendon MK, Apicella MA, Jones BD. Lindemann SR, et al. Infect Immun. 2007 Jun;75(6):3178-82. doi: 10.1128/IAI.01811-06. Epub 2007 Mar 5. Infect Immun. 2007. PMID: 17339345 Free PMC article. - Regulation of virulence gene transcripts by the Francisella novicida orphan response regulator PmrA: role of phosphorylation and evidence of MglA/SspA interaction.
Bell BL, Mohapatra NP, Gunn JS. Bell BL, et al. Infect Immun. 2010 May;78(5):2189-98. doi: 10.1128/IAI.00021-10. Epub 2010 Mar 15. Infect Immun. 2010. PMID: 20231408 Free PMC article. - Role of peroxiredoxin of the AhpC/TSA family in antioxidant defense mechanisms of Francisella tularensis.
Alharbi A, Rabadi SM, Alqahtani M, Marghani D, Worden M, Ma Z, Malik M, Bakshi CS. Alharbi A, et al. PLoS One. 2019 Mar 14;14(3):e0213699. doi: 10.1371/journal.pone.0213699. eCollection 2019. PLoS One. 2019. PMID: 30870480 Free PMC article. - Francisella tularensis Confronts the Complement System.
Brock SR, Parmely MJ. Brock SR, et al. Front Cell Infect Microbiol. 2017 Dec 19;7:523. doi: 10.3389/fcimb.2017.00523. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 29312899 Free PMC article. - Global transcriptional response to mammalian temperature provides new insight into Francisella tularensis pathogenesis.
Horzempa J, Carlson PE Jr, O'Dee DM, Shanks RM, Nau GJ. Horzempa J, et al. BMC Microbiol. 2008 Oct 8;8:172. doi: 10.1186/1471-2180-8-172. BMC Microbiol. 2008. PMID: 18842136 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous